5.1.2B Sensory responses to chemicals
The electrophysiological response of an insect to
semiochemicals can be studied with the electroantennogram (EAG) of
the whole antenna or the single-cell technique that measures
responses of specific receptor cells (Payne, 1979). Each antennal
receptor cell contains multiple acceptor sites that interact with
the chemicals. Bark beetle olfactory cells on the antennae have
been shown to be of several functional types, which probably are
found in every species: (1) a highly specific type such as the
ipsdienol-sensitive cells in I. paraconfusus and I. pini that is
responsive only to one of two possible enantiomers (cannot be
superimposed but otherwise identical structures, see alpha-pinene in
Fig. 4),

Fig. 4. Major monoterpenes of conifers. Note that the enantiomers
of alpha-pinene are identical except that they are non-superimposable
(mirror images). Camphene, B-pinene, 3-carene, B-phellandrene, and
limonene also have two enantiomers, although only (-)-B-pinene and
(+)-3-carene are found in trees (Mirov, 1961). Myrcene and
terpinolene are achiral.
(2) a pheromone-sensitive type that is also responsive to
some other synergists or inhibitors such as the frontalin cells of
D. frontalis (the cells have at least two acceptor types each
specific for one enantiomer of frontalin, Fig. 5), and (3)
"generalist" types that respond to host monoterpenes as well as
pheromones to some extent (Mustaparta et al., 1980; Payne et al.,
1982; Dickens et al., 1985; Dickens, 1986).
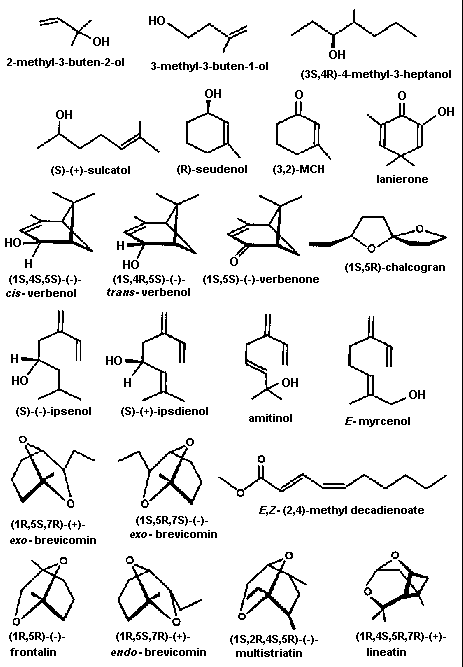
Fig. 5. Pheromone components of bark beetles. Key: aggregation
component (a), inhibitor of aggregation (i). Row 1: 2-methyl-3-
buten-2-ol (a, I. typographus); 3-methyl-3-buten-1-ol (a, I.
cembrae), 4-methyl-3-heptanol (a, S. multistriatus). Row 2:
sulcatol (a, G. sulcatus, G. retusus); seudenol (D. pseudotsugae,
D. rufipennis, D. simplex); MCH (i, D. pseudotsugae); lanierone (a,
I. pini). Row 3: cis-verbenol (a, I. paraconfusus, I. typographus,
I. calligraphus); trans-verbenol (a, D. ponderosae, T. minor; i, D.
brevicomis); verbenone (i, Dendroctonus); chalcogran (a,
Pityogenes). Row 4: ipsenol (a, Pityokteines curvidens, and many
Ips: e.g. I. paraconfusus, I. grandicollis), ipsdienol (a, many
Ips, e.g. I. paraconfusus, I. duplicatus, I. pini, I. calligraphus,
I. avulsus); amitinol (a, I. amitinus); E-myrcenol (a, I.
duplicatus). Row 5: (+)-exo-brevicomin (a, D. brevicomis,
Dryocoetes); (-)-exo-brevicomin (a, Dryocoetes); methyl
decadienoate (P. chalcographus). Row 6: frontalin (a, many
Dendroctonus); endo-brevicomin (i, D. frontalis); multistriatin (a,
S. multistriatus); lineatin (a, T. lineatum). References to above
pheromones are in reviews by Borden (1982) and Byers (1989a), and
the following (Bakke, 1975; Baker et al., 1977; Lanne et al., 1987;
Borden et al., 1987; Byers et al., 1989b, 1990a, b; Teale et al.,
1991; Camacho et al., 1993).
The technique of "differential adaptation" first attenuates an
electrophysiological response by exposing a receptor cell (or
antenna) to a high concentration of a compound until specific
acceptor sites are "saturated", then these sites are exposed to a
different volatile to see if any responses are elicited (Payne and
Dickens, 1976; Payne, 1979; Dickens et al., 1985). By using this
technique with single-cell recordings it was shown that D.
pseudotsugae has at least four olfactory cell types (Dickens et
al., 1985). Three types are each most sensitive to either MCH,
seudenol, or frontalin (Fig. 5), although they are all stimulated
somewhat by all of these pheromone components. The fourth cell type
is most sensitive to host compounds released by excavating beetles
that are synergists of pheromone components. Acceptors can be
specific for one enantiomer of a chiral mixture (Payne et al.,
1983) and there may be either only one type of acceptor per cell
(e.g. (+)- or (-)-ipsdienol in I. paraconfusus (Mustaparta et al.,
1980) or both types of chiral acceptors on the same cell (e.g. (+)-
and (-)-frontalin in D. frontalis (Payne et al., 1982) and D.
pseudotsugae (Dickens et al., 1985).
The antennae of both sexes of I. paraconfusus are equally
sensitive (EAG) to natural pheromone and to (+)-ipsdienol (Light
and Birch, 1982). However, the males have been shown to be
relatively less attracted by higher concentrations of synthetic
pheromone components, and they were not as likely to fly directly
toward the pheromone source as were females (Byers, 1983a). Thus,
the sexual differences in behavioral response of I. paraconfusus to
aggregation pheromone appear to be the result of differences in
central nervous system (CNS) integration rather than differences in
peripheral receptors. Cis-verbenol (Fig. 5) elicits a similar
electrophysiological dose-response curve for both sexes of I.
typographus, but some sexual differences exist for response to
methyl butenol (Dickens, 1981). Similar to I. paraconfusus, males
of I. typographus orient less directly to pheromone at the final
landing on the host than females (Schlyter et al., 1987c); this
behavioral difference could be due either to CNS differences
between the sexes or to the lesser receptor sensitivity of males to
methyl butenol. Mustaparta et al. (1980) have shown that eastern US
populations of I. pini have separate cells for each of the two
ipsdienol enantiomers that are synergistic attractants (Lanier et
al., 1980). However, the exposure of the two cells to the
enantiomers together did not synergistically increase the nerve
impulse rate, and so it was concluded that synergism in this case
acts at the CNS level.
Of the three or four receptor types found in most species it
appears that individual receptors within a type also can vary in
their response spectrum to various chemicals (Dickens, 1986;
Dickens et al., 1985; Mustaparta et al., 1980, 1984; Tommerås et
al., 1984). In the few cases so far known, the cell responsive to
host-plant chemicals are present in both sexes, but the host-
selecting sex (males of Ips or females of Dendroctonus) has a lower
threshold to plant compounds (Dickens, 1981, 1986; Dickens et al.,
1983). However, the role of plant compounds in long-range
orientation of many species of Ips is not certain (as discussed in
part 5.2). Probably short-range behaviors on the bark (e.g.,
gustatory responses) are influenced by host volatiles, but this
also is poorly understood.
Some bark beetles have receptors sensitive to compounds they
do not produce but are found in several other bark beetles as
pheromone components (Tommerås et al., 1984). Lanne et al. (1987)
showed compounds not found in T. piniperda (exo-brevicomin,
ipsenol, and pheromone components of other bark beetle species)
elicited an EAG response in the beetle. Some of these compounds are
found in competing species of bark beetles, but other compounds are
probably found only in species colonizing nonhost trees (e.g.,
Norway spruce). Three sympatric species of the southern U.S., Ips
avulsus, I. calligraphus, and I. grandicollis, are most responsive
electophysiologically to components of their own pheromones
(ipsenol, ipsdienol, cis-verbenol); but they can also respond to
components of their sibling species as well as frontalin and
verbenone from D. frontalis and alpha-pinene from their host pine
released by activities of other species (Smith et al.,
1988).
The difficulties with electrophysiological methods for
unraveling ecological phenomena during host finding and tree
colonization are three-fold: (1) the electrical measurements may
not be correlated to the behavior of interest, (2) the nerve
impulses vary between cell types (Mustaparta et al., 1980) of which
all are not located and tested, and (3) the electrical patterns are
further integrated in the complexity of the CNS.

Byers, J.A. 1995. Host tree chemistry affecting colonization in bark
beetles, in R.T. Cardé and W.J. Bell (eds.). Chemical Ecology of
Insects 2. Chapman and Hall, New York, pp. 154-213.

