Department of Entomological Sciences, University, of Califomia, Berkeley, California 94720
*Present address:

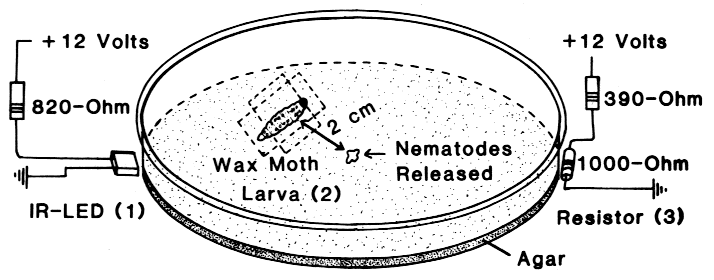
| TABLE 1. Aggregation of N. carpocapsae at the periphery of an agar-filled petri dish in areas which had been heated above the ambient temperature (19o C) by various heat sources under either light or dark conditions | |||
| Heat source | At each heated area | ||
|---|---|---|---|
| In light room | Temperature above ambient | Numbers aggregating | Number at 10 non-heated areas |
| IR-LED, 1 mm away1 | 0.08oC | 22, 3, 10*) | 1, rest 0 |
| IR-LED, touching2 | 0.3oC | 21, 13*) | 1, 1, rest 0 |
| 1,000-ohm resistor3 | 0.9oC | 23, 33*) | 1, rest 0 |
| In dark room: | |||
| IR-LED, touching2 | 0.3oC | 21, 41*) | 1, rest 0 |
| 1,000-ohm resistor3 | 0.9oC | 45, 28*) | 1, 1, rest 0 |
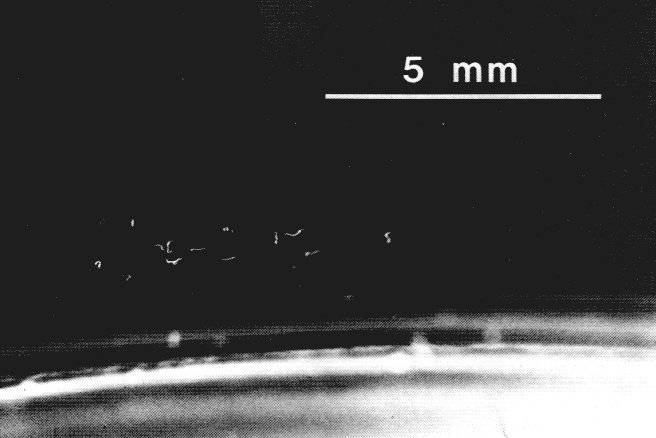
| TABLE 2. Number of N. carpocapsae aggregating at the periphery of an agar-filled petri dish in areas which were heated about 1o C above various ambient temperatures in the dark | ||
| Ambient temperature | 1,000-ohm Resistor1 | Control areas average of 10 |
|---|---|---|
| 12.5oC | 18 (13, 23)2)*) | 0.3 |
| 17.0oC | 60.5 (46, 75)*) | 0 |
| 20.0oC | 46.5 (40, 53)*) | 0.1 |
| 24.3oC | 57.0 (46, 75)*) | 0.1 |
| 26.5oC | 3.25.5 (0, 2, 3, 8)*) | 0.1 |
|
JOHN A. BYERS* & GEORGE O. POINAR Jr. Department of Entomological Sciences, University, of Califomia, Berkeley, California 94720 *Present address: 
|
|---|