Byers, J.A. 2002. Internet programs for drawing moth pheromone analogs and searching literature database.
Journal of Chemical Ecology 28:807-817.  pdf
pdf
Abstract--
An Internet web page is described for organizing and analyzing information about lepidopteran sex pheromone components.
Hypertext markup language (HTML) with JavaScript program code is used to draw moth pheromone analogs by combining GIF bitmap
images for viewing by
 web browsers such as Netscape or Microsoft Internet Explorer. Straight-chain hydrocarbons of 5-22 carbons with epoxides
or unsaturated positions of E- or Z- geometrical configuration with several alternative functional groups
can be drawn by
simply checking menu bars or checkboxes representing chain length, E/Z unsaturation points, epoxide position and
chirality, and optional functional groups. The functional group can be an aldehyde, alcohol, or ester of formate,
acetate, propionate, or butyrate. The program is capable of drawing several million structures and naming them
[e.g., (E,E)-8,10-dodecadien-1-ol and abbreviated as E8E10-12:OH]. A Java applet program run from the same page
searches for the presently drawn structure in an internal database compiled from the Pherolist, and if the component is
found, provides a textarea display of the families and species using the component. Links are automatically specified for
drawn components if found in the Pherolist web site (maintained by H. Arn). Windowed links can also be made to two
other JavaScript programs that allow searches of a web site database with over 5900 research citations on lepidopteran
semiochemicals and a calculator of vapor pressures of some moth sex pheromone analogs at a specified temperature.
Various evolutionary and biosynthetic aspects are discussed in regard to the diversity of moth sex pheromone components.
web browsers such as Netscape or Microsoft Internet Explorer. Straight-chain hydrocarbons of 5-22 carbons with epoxides
or unsaturated positions of E- or Z- geometrical configuration with several alternative functional groups
can be drawn by
simply checking menu bars or checkboxes representing chain length, E/Z unsaturation points, epoxide position and
chirality, and optional functional groups. The functional group can be an aldehyde, alcohol, or ester of formate,
acetate, propionate, or butyrate. The program is capable of drawing several million structures and naming them
[e.g., (E,E)-8,10-dodecadien-1-ol and abbreviated as E8E10-12:OH]. A Java applet program run from the same page
searches for the presently drawn structure in an internal database compiled from the Pherolist, and if the component is
found, provides a textarea display of the families and species using the component. Links are automatically specified for
drawn components if found in the Pherolist web site (maintained by H. Arn). Windowed links can also be made to two
other JavaScript programs that allow searches of a web site database with over 5900 research citations on lepidopteran
semiochemicals and a calculator of vapor pressures of some moth sex pheromone analogs at a specified temperature.
Various evolutionary and biosynthetic aspects are discussed in regard to the diversity of moth sex pheromone components.
Key Words--Lepidoptera, moth pheromone components, olefinic, aliphatic, epoxides, aldehydes, alcohols, acetate esters, Internet, JavaScript, computer program, Java applet, database, vapor pressures
INTRODUCTION
During the last few years, the Internet has become accessible to most scientists throughout the world. The quantity of
information available through the Internet, or World Wide Web, is likely to grow, improve in quality, and become ever
more important to research productivity and education (Byers, 1999; Zenger and Walker, 2000). Web pages are composed of
hypertext markup language (HTML) code and are interpreted by web browsers such as Netscape Communicator and Microsoft
Internet Explorer (IE), accounting for 37% and 61%, respectively, of 9394 visitors to the menu page,
www.vsv.slu.se/cec/h.htm, (during 2000, eXTReMe Tracking). JavaScript is a language, originating from Netscape
Comm. Corp., that can be loaded from a web page and run by browsers to achieve a moderate level of interactivity and
data processing (Goodman, 1996). Java applets (Sun Microsystems Inc.) are separately compiled programs that are called
into action by the HTML or JavaScript code and run by the browser or associated software to yield a true program
environment, although with security constraints so that only the screen image can be manipulated (Lemay and Perkins,
1997; Vanhelsuwé et al., 1997; Cadenhead, 1999). The use of Java applet programs makes it possible to achieve full
interactivity and advanced graphical applications. However, by clever use of bitmapped images, JavaScript can also
produce interesting visual effects.
From 1975 to 1991 in the Journal of Chemical Ecology, there were 619 articles published on Lepidoptera which
accounted for 29% of the journal's reports (Byers, 1992). During the subsequent nine years, 1992-2000, this figure
dropped slightly to 25% of 1840 articles. For all insect pheromone papers (1993-2000), BIOSIS Previews
cites 3775 articles with 45.3% on Lepidoptera (nearly all on moths). Due to this large interest in lepidopteran
pheromones, it was desirable to make a web page that draws and names most moth pheromone components and analogs by
simply selecting checkboxes or buttons controlled by JavaScript code. I also wanted the web page to run a Java
applet that would immediately tell the user if the drawn compound was a known pheromone component of one or more species
in the Pherolist (Arn et al., 1992; Arn, 2000), the well-known web site covering moth pheromone components
(www-pherolist.slu.se/index.html). If a known component is drawn, then the page should allow the user to save time by
linking automatically to the specific component in the Pherolist. Another goal was to link the page to a searchable web
database on moth literature citations (Byers, 2000) and a calculator of moth analog vapor pressures. The final objective
was to discuss the diversity of double bond positions of moth sex pheromone components (Pherolist) and analogs
(program drawn) in regard to evolutionary and biosynthetic aspects. The web page is at the Internet address:
www.wcrl.ars.usda.gov/cec/java2/mothchem.htm (Figure 1).
METHODS AND MATERIALS
The JavaScript code for drawing moth pheromone analogs is found in web frames that can be viewed by Netscape
and IE by right-clicking on the frame and selecting "view source". Alternatively, the code for each frame can be viewed
from pop-up windows (three buttons at bottom of lower frame, Figure 1).
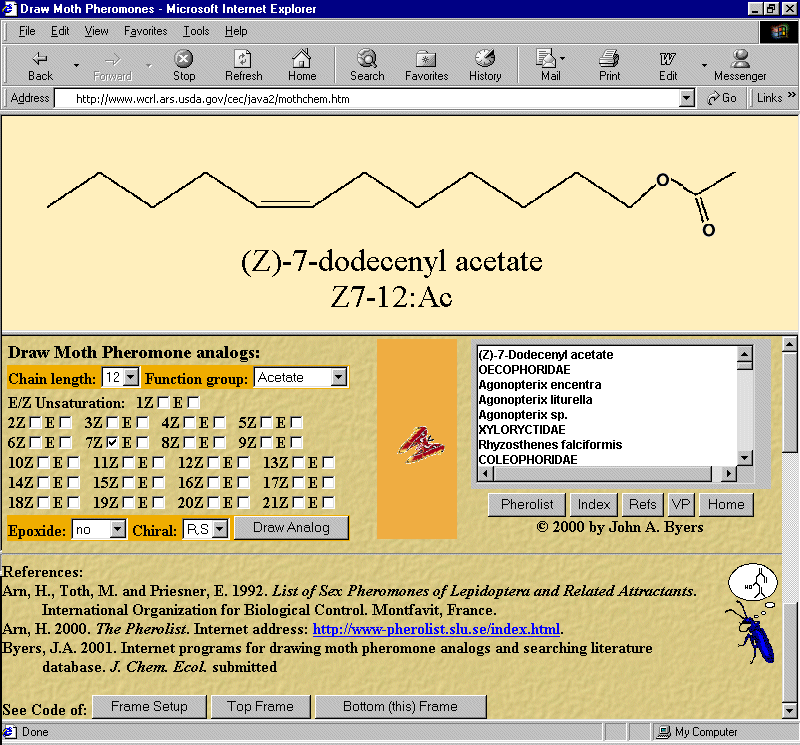
FIG. 1. Internet browser view of frames of web page that draws moth pheromone analogs in top frame based on user
input in the bottom frame. Functions of various buttons and textboxes are explained in the text.
To draw a molecule, the user selects a chain length from 5 to 22 from a drop-down menu with only one option
selection allowed (Figure 1, lower frame). The same input method is used for selecting the epoxide position and chirality
as well as the functional group: either none, an aldehyde, alcohol, or esters of formate, acetate, propionate, and
butyrate. A pair of checkboxes for each possible carbon position in the chain can be checked to designate geometrical
configuration (E or Z) of double bonds. Only one box of a pair can be checked. After input is selected, the
"draw analog" button is clicked. Several possible error messages may occur if an E/Z or epoxide position checkbox is
selected outside the chain length or is next to position 1 of a functional group. Furthermore, adjacent positions cannot
both be unsaturated nor can they be next to or within an epoxide, as these structures are chemically unlikely. Chains
with unsaturation at the end opposite the functional group cannot be designated E or Z (but are delta).
The program then takes the input data and converts them to variables whose values are used to control the drawing
of a molecule in the upper frame (Figure 1). The drawing is composed of an appropriate permutation of 22 bitmapped GIF
images with transparent backgrounds. These are scaled according to the viewer's screen resolution and displayed from
left to right such that the molecule is drawn from the highest numbered carbon toward the functional group. Six of the
GIF images represent carbon-carbon bonds, 6 are for epoxides, and 10 are for different configurations of the 6 possible
functional groups. The variables are also used to construct the IUPAC name, e.g. (E,E)-8,10-dodecadien-1-ol, and a short
name, E8E10-12:OH. A further abbreviated name, e.g. e8e1012oh, is made and searched for in an internal database array of
about 420 names. If found, as it would be in this case (codlemone), the abbreviation serves as the prefix of the HTML
file name in the Pherolist in order to allow automatic linking to this file. The 420-name internal database was made by
a BASIC program that extracted pheromone components (and female-produced analogs that could be drawn) from the 544 HTML
files (1.5 MB) concerning components that are in the Pherolist as of June 2000.
Another feature found at the right of the lower frame (Figure 1) is a Java applet that is represented by a
scrollable textarea. The applet consists of a database of component names and the corresponding insect families and
species that have or are attracted to the components as extracted from the Pherolist by the BASIC program. Initially the
textarea informs the user of the purpose of the textarea and gives credits to the Pherolist. The data of the applet
consists of an array of nonredundant names of families and species in no particular order, but simply as they were
encountered in the 544 HTML Pherolist files on chemicals. Each of these files has the component name, as found between
the beginning and ending HTML tags of the title, e.g. (E,E)-8,10-dodecadien-1-ol. Similarly, there are
unique text strings that indicate the families, genera and species reported to be attracted or produce the component.
However, the resulting extracted file was larger than 64K, the maximum size for a method in Java (version 1.2.2), so the
information was indexed using numbers for the array of nonredundant species/families mentioned above. This allowed 420
components to be compared to 1716 species and families in the Java applet. Thus, when the user draws a component, e.g.
(E,E)-8,10-dodecadien-1-ol, the Java applet searches its database and shows the names of all families and species
attracted by this component (e.g. 3 subfamilies of Tortricidae and 13 species).
Another feature is a button ("Refs") that when clicked allows the user to open a pop-up window with two frames
of a web database of citations on over 5900 papers of lepidopteran semiochemicals (Figure 2).
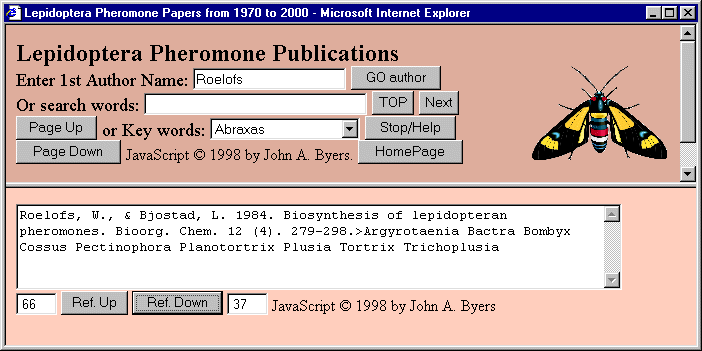
FIG. 2. Internet browser view of frames of a JavaScript database show a search for a specific citation from among
currently 5912 references on semiochemicals of Lepidoptera.
A BASIC program similar to
that reported earlier (Byers, 1992) reformatted the database which served as input to another BASIC program that fused
templet files of HTML and JavaScript code with the reformatted data to produce 92 web pages with self-searching
capability using JavaScript code and "cookies". Cookies are browser files maintained on the user's hard drive that store
information that normally would vanish when the user moved to another web page. None of the literature web pages has
more than 30K of data since it was found that data strings larger than 32K caused browsers to incompletely load the
page. To update the list of citations, the entire database must be reformatted by the BASIC program into 30K blocks such
that no first author is split between pages. The BASIC applications were programmed with Microsoft QuickBASIC version
4.5 while the Java applet (small application) was done with Sun Microsystems' 1.2.2 package.
Still another feature is a button ("VP") that displays a window with a JavaScript coded calculator of vapor
pressures of a few unsaturated acetate esters as well as saturated aldehydes, alcohols, hydrocarbons, and fatty acids
with 10 or more carbon chain lengths (Figure 3).
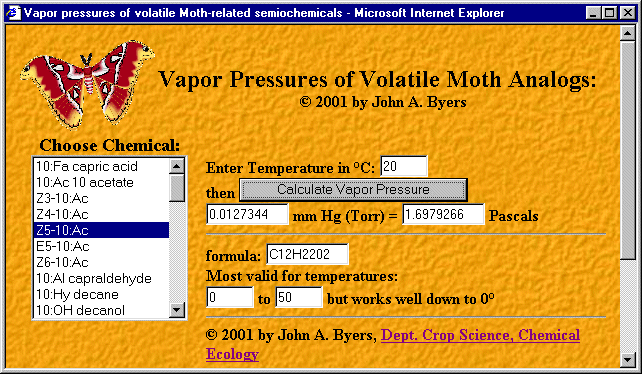
FIG. 3. Internet browser view of a pop-up window of a JavaScript web page for calculating vapor pressures of some moth
pheromone components and fatty acids at different temperatures.
A compound is selected from a pop-up menu on the left and a temperature
is entered on the right so that the vapor pressure (V) can be calculated from the equation, V = 10(-0.2185 A/K) + B,
where V is in mm mercury (Torrs), A is molar heat of vaporization in calories per gram mole, K is temperature in degrees
Kelvin, and B is a constant (Schlessinger, 1971). Exponential regression was used on the data of Olsson et al. (1983) to
obtain vapor pressure equations for some moth acetate esters. Vapor pressures can also be estimated from boiling points
at partial pressures according to the methods of Hass and Newton (1971).
RESULTS AND DISCUSSION
Operation of the web page for drawing unsaturated straight-chain hydrocarbons with various functional groups is
user-friendly since only two drop-down menu bars and possibly a few checkboxes need be selected to draw and name the
majority of natural moth pheromone components (Figure 1). The program can draw the 43 unbranched hydrocarbons with no
functional group, 71 epoxides, 82 aldehydes, 66 alcohols, 167 acetate esters, and 10 other esters listed in the Pherolist.
Thus, about 83% of the 528 currently listed components (produced or attractive) in the Pherolist can be drawn
(16 additional analogs containing chlorine, fluorine or nitrogen are considered unnatural). During the extraction of
compound names from the Pherolist, names with "methyl", "chlor", "fluor", "one", "oate", "yn", "2-ol", "iso", "nitr",
and "valer" were excluded to simplify the drawing function. Of the 71 epoxides known, all but one have one epoxide per
compound and these are all cis-configured being either of two chiral forms, (R,S) and (S,R), so it was possible to
include these components (Millar, 2000; Pherolist). However, the chiralities of about half of the aliphatic epoxides
(all cis-) have not been determined. Although chemists have made trans-configured epoxides, they have not been discovered
in moths (Millar, 2000; Pherolist). One important group that was excluded contains 48 methyl-branched analogs.
This group was not considered since the structures are often chiral and thus naming becomes more complex.
The remaining 49 excluded names were: "one" (16), "oate" (13), "2-ol" (8), "iso" (6), and "valer" (1).
The program also can draw several million unused or undiscovered analogs. For example, considering 14-carbon
acetate esters, there are 23 different singly unsaturated compounds, 200 doubly unsaturated ones, and 816 triply
unsaturated ones (E, Z, or delta) (Byers, unpublished). Even more compounds are possible when four (1568), five (1232)
and the maximum of six unsaturations (256) are considered. However, further unsaturation in a molecule would make it
less stable due to chemical alteration by hydrogenation or geometric isomerization. Natural selection would not favor
the evolution of a less stable pheromone component that otherwise would require continuous production of large
quantities to be effective. Naturally occurring longer chain hydrocarbons have been reported with up to three
unsaturations and rarely four, e.g. (Z,Z,Z,Z)-3,6,9,11-nonadecatetraene in Alsophila pometaria (Geometridae; Wong et
al., 1984) and (Z,Z,Z,Z)-7,13,16,19-docosatetraen-1-ol isobutyrate in Euproctis chrysorrhoea (Lymantiidae; Leonhardt
et al., 1991), but none have been found with five. Theoretically, 10 unsaturations can fit within a 22-carbon chain
acetate ester and be named using the program. An analysis of the 294 unbranched naturally occurring aldehydes,
acetate esters, and alcohols (Pherolist) shows that many possible permutations of unsaturations have not been
reported in the Lepidoptera. There were 125 components with one unsaturation (42%) and 130 components with two
unsaturations (44%). Of the latter, 77% have one single bond in between the unsaturations (conjugated), while 5%
have two single bonds between the unsaturations and 18% have more than two single bonds between. Only 16 components
(5%) have three unsaturations and none have four unsaturations (only a few straight chain hydrocarbons have four).
The number of different components reported in moths to date (about 528) appears strikingly few when compared
to the several million unsaturated E- and Z-analogs potentially available as sex pheromone components. For example,
there are 1039 possible 14-carbon chain acetate ester components with one, two or three unsaturations (E, Z or delta)(
Byers, unpublished data), but only 42 of these have been identified in moths (Pherolist; Byers, unpublished data). The limitation to
essentially 1039 possible analogs may indicate this is a sufficiently large number to allow any species to easily
evolve a unique blend from among these. However, there may not have been enough evolutionary time for speciation
events to create more divergence and use of more structures. The limitation to three or less unsaturations in
components may also be due to chemical constraints such as increased instability with desaturation.
The enzyme systems for desaturation and chain-shortening involved in moth component biosynthesis (Bjostad et
al., 1981; Roelofs and Wolf, 1988; Tillman et al., 1999) may have evolved in response to the inherent stability of
mono- and diunsaturated hydrocarbons as compared to less stable multiunsaturated alternatives. Probably the most
obvious hypothesis to account for the relatively few unsaturations in a moth component is that the fatty acid
precursors (oleic, linoleic, and linolenic acids) have one, two or three unsaturations, respectively. However, as mentioned
above, a few moths biosynthesize aliphatic chains with four unsaturations and many odd and even carbon bond positions
can be unsaturated by the (delta)5, 9, 10, 11, 12, and 14 desaturases and chain-shortening enzymes that have been
discovered so far (Bjostad et al., 1981; Roelofs and Wolf, 1988; Foster and Roelofs, 1988, 1996; Zhao et al.,
1990; Jurenka, 1997; Tillman et al., 1999). If there are constraints on the evolution of biosynthetic enzymes,
there could also be limits on the antennal receptors to discriminate between higher numbers of unsaturated
bonds (and constraints on the evolution of more complexity).
The limited numbers of unsaturated positions in moth analogs might also be due to some disadvantage in
using molecules with increased volatility due to higher numbers of unsaturated bonds. The double bond length
between carbons is shorter than in a single bond (also the H-C bonds are shorter on double bond carbons) which makes
the molecule smaller in area and, thus, more volatile (Morrison and Boyd, 1973). There are few data on vapor pressures
of moth acetate esters with multiple unsaturations. Olsson et al. (1983) found that Z9-14:Ac (16 carbons) has a
vapor pressure of 7E-4 mm Hg at 30.5°, which is similar to 14-carbon myristic acid [3E-4 mm (Schlessinger, 1971)].
Thus, comparing 18-carbon fatty acids as models for 18:Ac, the differences in vapor pressures between 18-carbon
fatty acids with none, one, two, or three unsaturations (stearic, oleic, linoleic, and linolenic acid) appear to be
only about 2.5-fold using methods of Schlessinger (1971) and Hass and Newton (1971). Moth species have used pheromone
components commonly with chain lengths from 18 down to 10, representing a decrease in volatility for saturated fatty
acids about 54-fold, for alcohols about 33-fold, and for aldehydes about 122-fold (Schlessinger, 1971; Hass and
Newton, 1971). It seems doubtful that the modest increases in volatility by increasing desaturation would prevent
evolution toward use of components with further unsaturation. However, no data were found to ascertain the effect
of adding more than three double bonds on vapor pressures of moth pheromone components.
Ultimately, there is no selection pressure or fitness benefit to making a pheromone component more complex
(more unsaturations or groups) once a unique species-specific structure has evolved. It is, however, apparently easier
to combine two simpler components into a unique blend than to increase the structural complexity of a single component
pheromone to achieve specificity. It is expected that there would be costs to maintain unnecessarily complex systems
for biosynthesis and reception when simpler ones suffice.
In moth pheromone components, two unsaturated bonds are always separated by at least one saturated bond, and
position one is never unsaturated in aldehydes, alcohols, and acetate esters due to a maximum of four carbon
bonds, keto-enol tautomerism, and dipole moments, respectively (Morrison and Boyd, 1973). Of the different moth
14-carbon acetate esters with two double bonds, the average number of saturated bonds separating two unsaturated
bonds is 1.7±0.66 (95% CL, N=20, Pherolist). Theoretically, if two unsaturated bonds in a 14-carbon chain acetate
ester are located in all possible ways such that they are separated by at least one single bond, then the average
number of single bonds separating unsaturated bonds would be four (calculated from BASIC code: FOR W = 10 TO 1
STEP -1: FOR X = 1 TO W: C = C + X: D = D + 1: NEXT: NEXT: AVE = C/D). This expected average separation distance of
four single bonds is much larger than the observed 1.7 bonds. Of the twenty 14:Ac components, 15 have one bond
separation (conjugated), three have more than 2 bonds, and only one has 2 bonds between unsaturations (as does the
fatty acid 9,12-linoleic acid).
It is also of interest to note that pheromone components and analogs have been identified from relatively few
moth species (1683 species from 50 families, Pherolist). In North America alone there are over 65 moth families (Borror
and DeLong, 1971) and the number of species worldwide is estimated to approach 185,000 (Holloway et al., 1987). The
limited number of species with identified pheromone components is probably because most work has focused on pests and
these species are a small fraction. Secondly, once certain components are known, their subsequent discovery in
other species is more likely because standards are more easily obtained and analytical methods for isolation and
identification have already been developed. For example, an unknown exotic structure might not pass through the gas
chromatographic columns in use, might occur in trace amounts, or have no comparable GC-MS standards (for identification) or
commercial sources (for field bioassay).
The web site, linked closely with the Pherolist and reference database, should provide an educational experience
for learning about moth pheromone components and aid in research. If a drawn pheromone component is on the Pherolist
and the "Pherolist" button is clicked, a pop-up window links automatically to the compound's web page in the
Pherolist. Another button ("Index") pops up a window displaying the main index of compounds of the Pherolist. As
mentioned earlier, the button "Refs" opens a window of a database of over 5900 citations on lepidopteran semiochemicals
and this page links to several other citation databases on semiochemicals of Coleoptera (2256 citations),
Hymenoptera (1724 citations), and other insects (2348 citations). The vapor pressure calculator ("VP" button) can aid
in predicting appropriate release rates of components in the lab and field in ratios depending on the mole percent in
solution times the vapor pressure. For example, if components A:B have a mole percent ratio of 1:10 in solution and
vapor pressure ratios of 1:100, then component B will have a 1000-fold higher release rate than A.
Acknowledgments--
The study was supported in part by a grant from the Swedish Council for Forestry and Agricultural Research (SJFR).
I am grateful to Heinrich Arn for introducing me to the Pherolist during his visits to Sweden. Ashraf El-Sayed,
Miryan Coracini, Göran Birgersson, and Marie Bengtsson provided helpful advice.
JOHN A. BYERS1
Department of Crop Science, Swedish University of Agricultural Sciences, SE-230 53 Alnarp, Sweden
1Present address: Western Cotton Research Laboratory, USDA-ARS, 4135 E. Broadway Road, Phoenix, Arizona 85040.
|
|---|
REFERENCES
ARN, H. 2000. The Pherolist. Internet address: http://www-pherolist.slu.se/index.html.
ARN, H., TOTH, M., and PRIESNER, E. 1992. List of Sex Pheromones of Lepidoptera and Related Attractants.
International Organization for Biological Control. Montfavit, France.
BJOSTAD, L.B., WOLF, W.A., and ROELOFS, W.L. 1981. Total lipid analysis of the sex pheromone gland of the redbanded
leafroller moth, Argyrotaenia velutinana, with reference to pheromone biosynthesis. Insect Biochem. 11, 73-79.
BORROR, D.J., and DELONG, D.M. 1971. An Introduction to the Study of Insects. Holt, Reinhart and Winston. New york.
BYERS, J.A. 1992. Trends in chemical ecology revealed with a personal computer program for searching data bases of
scientific references and abstracts. J. Chem. Ecol. 18:1481-1495.
BYERS, J.A. 1999. Interactive learning using expert system quizzes on the internet. Educat. Media Int. 36:191-194.
BYERS, J.A. 2000. Moth pheromone component literature. Internet address: http://www.vsv.slu.se/johnb/java/leppap/leppap.htm.
CADENHEAD, R. 1999. Sams Teach Yourself Java 2 in 24 Hours. Sams Publishing.
FOSTER, S.P., and ROELOFS, W.L. 1988. Sex pheromone biosynthesis in the leafroller moth Planotortrix excessana by (delta)desaturation. Arch. Insect Biochem. Physiol. 8:1-9.
FOSTER, S.P., and ROELOFS, W.L. 1996. Sex pheromone biosynthesis in the Tortricid moth, Ctenopseustis herana (Felder and Rogenhofer). Arch. Insect Biochem. Physiol. 32:135-147.
GOODMAN, D. 1996. Danny Goodman's JavaScript Handbook. IDG Books Worldwide, Inc.
HASS, H.B., and NEWTON, R.F. 1971. Correction of boiling points to standard pressure. pp. D-144-145, in R.C. Weast (ed.). Handbook of Chemistry and Physics. The Chemical Rubber Co., Cleveland, Ohio, USA.
HOLLOWAY, J.D., BRADLEY, J.D., and CARTER, D.J. 1987. Lepidoptera. pp. 1-22, in C.R. Betts (ed.). CIE Guides to Insects of Importance to Man. CAB International Inst. Entomol., British Mus. Nat. Hist., Oxford, UK.
JURENKA, R.A. 1997. Biosynthetic pathway for producing the sex pheromone component (Z,E)-9,12-tetradecadienyl acetate in moths involves a (delta)12 desaturase. Cell. Mol. Life Sci. 53:501-505.
LEMAY, L., and PERKINS, C.L. 1997. Teach Yourself Java 1.1 in 21 Days. 2d Ed. Sams.net Publishing.
LEONHARDT, B.A., MASTRO, V.C., SCHWARZ, M., TANG, J.D., CHARLTON, R.E., PELLEGRINI-TOOLE, A., WARTHEN, J.D., Jr., SCHWALBE, C.P., & CARDÉ,
R.T. 1991. Identification of sex pheromone of browntail moth Euproctis chrysorrhoea L. (Lepidoptera: Lymantriidae). J. Chem. Ecol. 17:897-910.
MILLAR, J.G. 2000. Polyene hydrocarbons and epoxides: A second major class of lepidopteran sex attractant pheromones. Ann. Rev. Entomol. 45:575-604.
MORRISON, R.T., and BOYD, R.N. 1973. Organic Chemistry. Allyn and Bacon, Inc., Boston, Massachusetts, USA.
OLSSON, A.M., JÖNSSON, J.Å., THELIN, B., and LILJEFORS, T. 1983. Determination of the vapor pressures of moth
sex pheromone components by a gas chromatographic method. J. Chem. Ecol. 9:375-385.
ROELOFS, W.L., and WOLF, W.A. 1988. Pheromone biosynthesis in Lepidoptera.
J. Chem. Ecol. 14:2019-2031.
SCHLESSINGER, G.G. 1971. Vapor pressures, critical temperatures and critical pressures of organic compounds. pp. D-151-170, in R.C. Weast (ed.). Handbook of Chemistry and Physics. The Chemical Rubber Co., Cleveland, Ohio, USA.
TILLMAN, J.A., SEYBOLD, S.J., JURENKA, R.A., and BLOMQUIST, G.J. 1999. Insect pheromones - an overview of biosynthesis and endocrine regulation. Insect Biochem. Molec. Biol. 29:481-514.
VANHELSUWÉ, L., PHILLIPS, I., HSU, G-T., SANKAR, K., RIES, E., HELLER, P., McGLOUGHLIN, J., and ZUKOWSKI, J. 1997. Mastering Java 1.1. 2d Ed. SYBEX, San Francisco.
WONG, J.W., PALANISWAMY, P., UNDERHILL, E.W., STECK, W.F., and CHISHOLM, M.D. 1984. Novel sex pheromone components from
the fall cankerworm moth, Alsophila pometaria. J. Chem. Ecol. 10:463-473.
ZENGER, J.T., and WALKER, T.J. 2000. Impact of the Internet on entomology teaching and research. Annu. Rev. Entomol. 45:747-767.
ZHAO, C.-H., LÖFSTEDT, C., and WANG, X. 1990. Sex pheromone biosynthesis in the Asian corn borer Ostrinia furnacalis (II): Biosynthesis of (E)- and (Z)-12-tetradecenyl acetate involves (delta)desaturation. Arch. Insect Biochem. Physiol. 15:57-65.
 web browsers such as Netscape or Microsoft Internet Explorer. Straight-chain hydrocarbons of 5-22 carbons with epoxides
or unsaturated positions of E- or Z- geometrical configuration with several alternative functional groups
can be drawn by
simply checking menu bars or checkboxes representing chain length, E/Z unsaturation points, epoxide position and
chirality, and optional functional groups. The functional group can be an aldehyde, alcohol, or ester of formate,
acetate, propionate, or butyrate. The program is capable of drawing several million structures and naming them
[e.g., (E,E)-8,10-dodecadien-1-ol and abbreviated as E8E10-12:OH]. A Java applet program run from the same page
searches for the presently drawn structure in an internal database compiled from the Pherolist, and if the component is
found, provides a textarea display of the families and species using the component. Links are automatically specified for
drawn components if found in the Pherolist web site (maintained by H. Arn). Windowed links can also be made to two
other JavaScript programs that allow searches of a web site database with over 5900 research citations on lepidopteran
semiochemicals and a calculator of vapor pressures of some moth sex pheromone analogs at a specified temperature.
Various evolutionary and biosynthetic aspects are discussed in regard to the diversity of moth sex pheromone components.
web browsers such as Netscape or Microsoft Internet Explorer. Straight-chain hydrocarbons of 5-22 carbons with epoxides
or unsaturated positions of E- or Z- geometrical configuration with several alternative functional groups
can be drawn by
simply checking menu bars or checkboxes representing chain length, E/Z unsaturation points, epoxide position and
chirality, and optional functional groups. The functional group can be an aldehyde, alcohol, or ester of formate,
acetate, propionate, or butyrate. The program is capable of drawing several million structures and naming them
[e.g., (E,E)-8,10-dodecadien-1-ol and abbreviated as E8E10-12:OH]. A Java applet program run from the same page
searches for the presently drawn structure in an internal database compiled from the Pherolist, and if the component is
found, provides a textarea display of the families and species using the component. Links are automatically specified for
drawn components if found in the Pherolist web site (maintained by H. Arn). Windowed links can also be made to two
other JavaScript programs that allow searches of a web site database with over 5900 research citations on lepidopteran
semiochemicals and a calculator of vapor pressures of some moth sex pheromone analogs at a specified temperature.
Various evolutionary and biosynthetic aspects are discussed in regard to the diversity of moth sex pheromone components.


