I'm preparing to climb a ponderosa pine
tree and put synthetic pheromone components on the trunk (baiting).
I'm preparing to climb a ponderosa pine
tree and put synthetic pheromone components on the trunk (baiting). This tree was baited with synthetic D. brevicomis pheromone (E,F,M) so it would be
attacked by the beetles and then sawed into logs for use in tests described here.
This tree was baited with synthetic D. brevicomis pheromone (E,F,M) so it would be
attacked by the beetles and then sawed into logs for use in tests described here. These large sticky-traps
were built by criss-crossing two (76 X 203 cm) vinyl-coated flberglass
screens (Browne, 1978) coated with Stikem Special® (Mickel and Pelton,
Emeryville, California) over 4 (1.54 m long) stakes driven vertically into the
ground in such a way as to form a box 76 cm wide X 67 cm high.
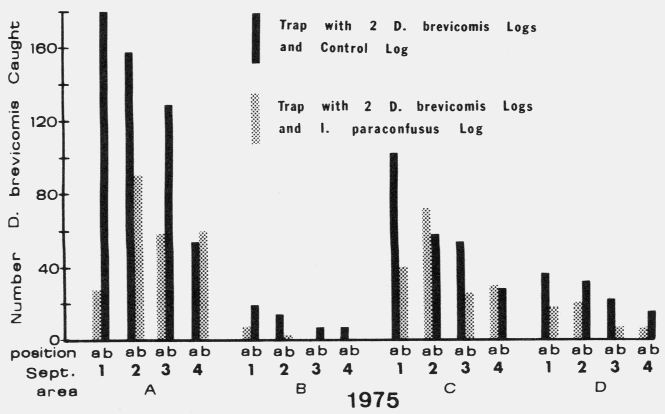
These large sticky-traps
were built by criss-crossing two (76 X 203 cm) vinyl-coated flberglass
screens (Browne, 1978) coated with Stikem Special® (Mickel and Pelton,
Emeryville, California) over 4 (1.54 m long) stakes driven vertically into the
ground in such a way as to form a box 76 cm wide X 67 cm high.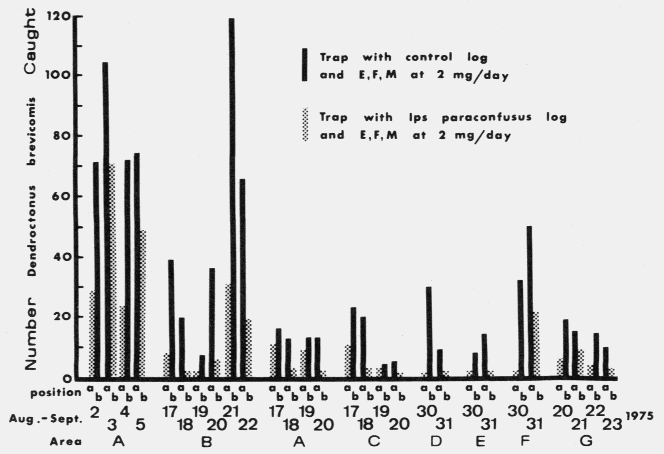
| TABLE 1. Inhibition of response of D. brevicomis and I. paraconfusus to natural and synthetic pheromone by verbenone or volatiles from infested logs | |||||
| Sex | Inhibition P valueb (total) | ||||
|---|---|---|---|---|---|
| Treatment vs. control | No caught (male:female) | Sex ratio (male:female) | Male Female | Same day Successive day | Female > Malec |
| Inhibition of D. brevicomis | |||||
| D. brevicomis, | 463 | 0.84 | |||
| I. paraconfusus | 212:251 | (0.70-1.00)a | |||
| 0.007 | 0.002 | ||||
| vs. | 0.31 | ||||
| 0.010 | 0.001 | ||||
| D. brevicomis | 896 | 0.93 | |||
| 432:464 | (0.82-1.06) | ||||
| - | |||||
| EFM, | 329 | 1.02 | |||
| I. paraconfusus | 166:163 | (0.82-1.26) | |||
| < 0.001 | < 0.001 | ||||
| vs. | 0.13 | ||||
| < 0.001 | < 0.001 | ||||
| EFM | 928 | 0.87 | |||
| 433:495 | (0.77-0.96) | ||||
| - | |||||
| Inhibition of I. paraconfusus | |||||
| I. paraconfusus, | 899 | 0.51 | |||
| D. brevicomis | 305:595 | (0.45-0.59) | |||
| 0.010 | 0.004 | ||||
| vs. | 0.008 | ||||
| 0.003 | 0.015 | ||||
| I. paraconfusus | 2,622 | 0.33 | |||
| 651:1,971 | (0.30-0.36) | ||||
| - | |||||
| I. paraconfusus, | 1, 156 | 0.20 | |||
| EFM | 194:962 | (0.17-0.24) | |||
| 0.960 | 0.390 | ||||
| vs. | - | ||||
| 0.290 | 0.750 | ||||
| I. paraconfusus | 1,283 | 0.20 | |||
| 214:1,069 | (0.17-0.23) | ||||
| - | |||||
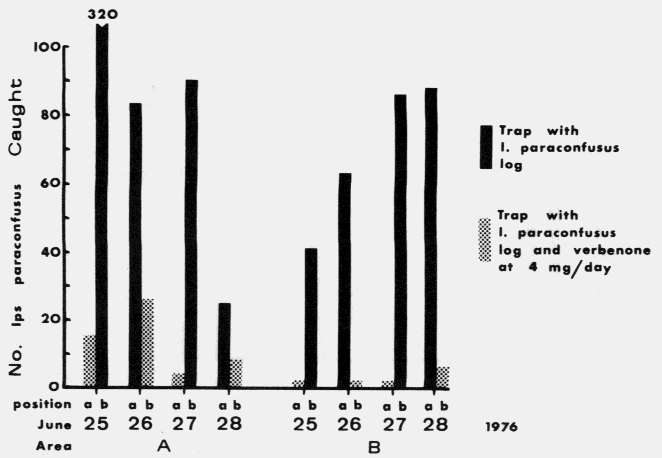
| I. paraconfusus, | 67 | 0.34 | |||
| verbenone | 17:50 | (0.20-0.59) | |||
| 0.036 | 0.012 | ||||
| vs. | 0.036 | ||||
| 0.012 | 0.012 | ||||
| I. paraconfusus | 798 | 0.13 | |||
| 91:707 | (0.10-0.16) | ||||
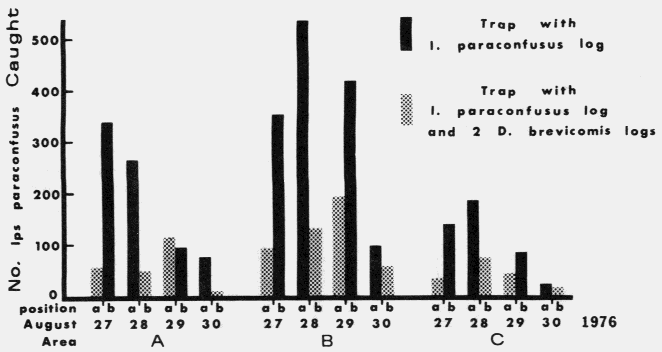
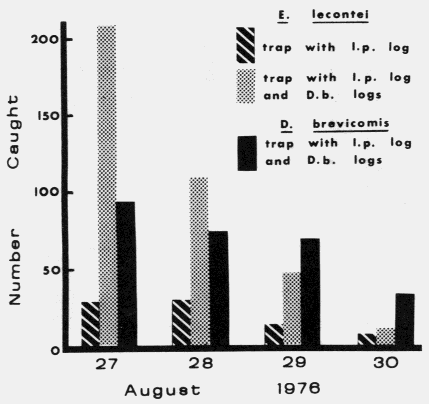
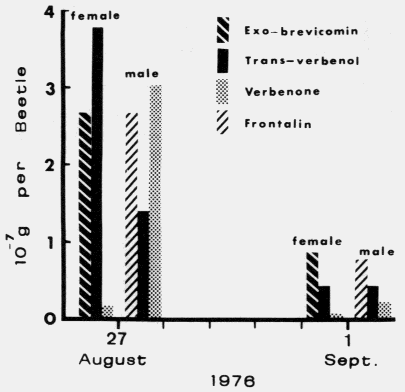
 Actual logs from which D. brevicomis
beetles were dissected and pheromone components quatified as shown in Figure 6 below.
Actual logs from which D. brevicomis
beetles were dissected and pheromone components quatified as shown in Figure 6 below.