 Stridulatory organ on back of head of female Ips paraconfusus showing the diffraction pattern of the rainbow.
Stridulatory organ on back of head of female Ips paraconfusus showing the diffraction pattern of the rainbow.
| TABLE 1. Catch of Ips paraconfusus and Enoclerus lecontei on 20 paired traps baited with logs infested with male and with male and female I. paraconfusus (August 26 - September 2, 1978) in the Sierra National Forest | ||||
| Catch | ||||
|---|---|---|---|---|
| I. paraconfusus | ||||
| Sticky traps containing | E. lecontei | Male | Female | Sex ratio (BCL)a |
| 30 male log plus blank log | 231 | 176 | 492 | 279b (2.35 - 3.32) |
| 30 male log plus 30 male + 90 female log | 291 | 115 | 405 | 3.52b (2.87 - 4.33) |
| P value of inhibitionc | 0.253 | 0.039 | 0.451 | |
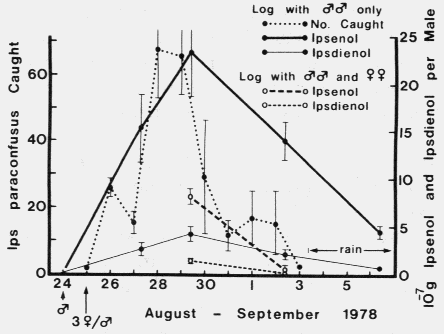 FIG. 1. Ipsenol and ipsdienol produced in mated and unmated male l. paraconfusus
during feeding in logs and the average number of I. paraconfusus attracted to traps
with logs infested with 30 unmated males in the Sierra National Forest. Brackets
represent ± SEX.
FIG. 1. Ipsenol and ipsdienol produced in mated and unmated male l. paraconfusus
during feeding in logs and the average number of I. paraconfusus attracted to traps
with logs infested with 30 unmated males in the Sierra National Forest. Brackets
represent ± SEX.| TABLE 2. Comparison of linear regrssion lines of quantities of ipsenol and ipsdienol (Y X 10-7 g/male) in hindguts of male Ips paraconfusus feeding in logs with or without females 4-12 days (X) after introduction of femalesa | ||||
| P valueb | ||||
|---|---|---|---|---|
| Equation | r2 | Slope difference | Elevation difference | |
| Ipsenol - male only log | Y = -2.35X + 32.7 | 0.87 | ||
| 0.62 | < 0.001 | |||
| Ipsenol - male with 3 females log | Y = -1.92X + 15.9 | 0.97 | ||
| Ipdienol - male only log | Y = -0.44X + 5.9 | 0.86 | ||
| 0.48 | < 0.001 | |||
| Ipsenol - male with 3 females log | Y = -0.32X + 2.6 | 0.95 | ||
| Ipsenol - male only log | Y = -2.35X + 32.7 | 0.95 | ||
| 0.003 | 0.001 | |||
| Ipsdienol - male only log | Y = -0.44X + 5.9 | 0.86 | ||
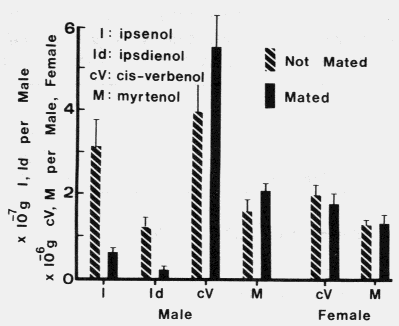 FIG. 2. Ipsenol, ipsdienol, cis-verbenol, and myrtenol produced in mated, fed males
and in unmated, emergent males and females exposed to myrcene and (-)-alpha-pinene
vapors (August 25,1979). Brackets above the bars represent+ SEX. A t-test showed
that unmated males produced significantly more ipsenol (P = 0.007) and ipsdienol
(P = 0.005) than mated males. There were no significant differences in the production
of either cis-verbenol or myrtenol between mated and unmated males or between
mated and unmated females (P > 0.1).
FIG. 2. Ipsenol, ipsdienol, cis-verbenol, and myrtenol produced in mated, fed males
and in unmated, emergent males and females exposed to myrcene and (-)-alpha-pinene
vapors (August 25,1979). Brackets above the bars represent+ SEX. A t-test showed
that unmated males produced significantly more ipsenol (P = 0.007) and ipsdienol
(P = 0.005) than mated males. There were no significant differences in the production
of either cis-verbenol or myrtenol between mated and unmated males or between
mated and unmated females (P > 0.1).
