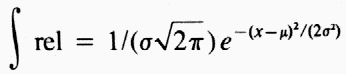
 Sticky-traps for catching Dendroctonus brevicomis, the western pine beetle, in ponderosa pine forest habitat
near Oakhurst (and Yosemite), California.
Sticky-traps for catching Dendroctonus brevicomis, the western pine beetle, in ponderosa pine forest habitat
near Oakhurst (and Yosemite), California.
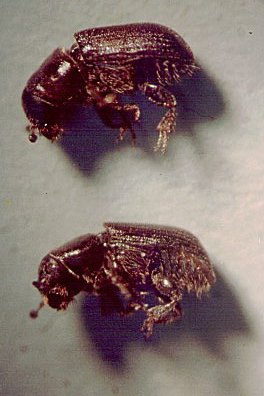
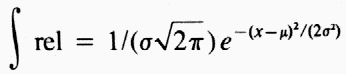
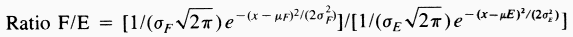
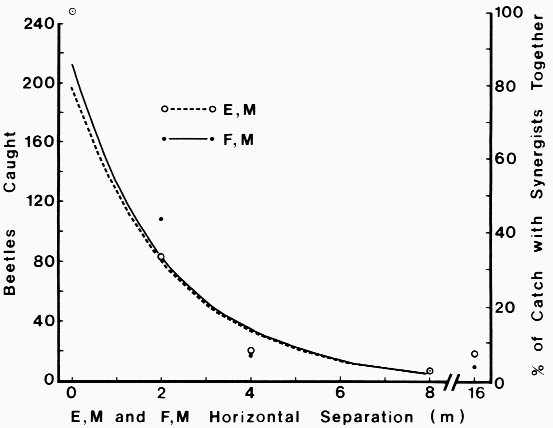
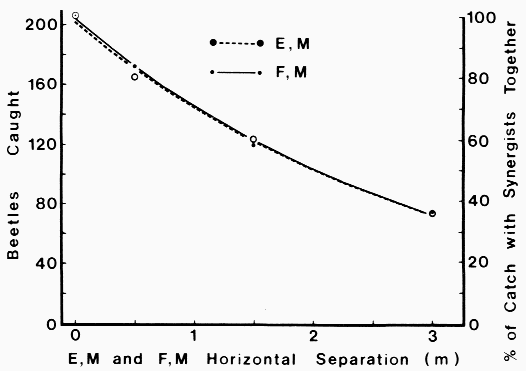
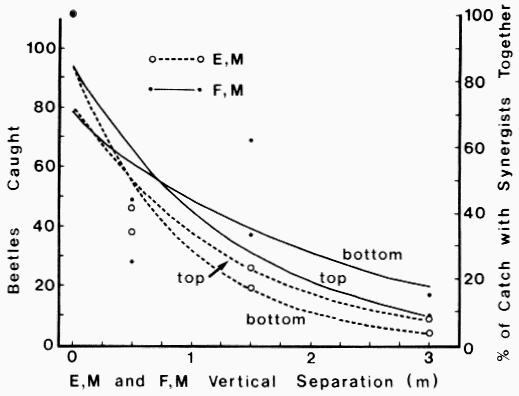
| Table 1. Sex ratios of catch of Dendroctonus brevicomis on horizontal or vertical trap pairs spaced different distances apart and containing pheromone components F, M (Frontalin + Myrcene) or E, M (exo-Brevicomin + Myrcene) | ||||
| Horizontal traps | Vertical traps - 1985 | |||
|---|---|---|---|---|
| 1978 | 1985 | Bottom | Top | |
| Trap (separation distance, m) | Male/Female (95% BCL)a | Male/Female (95% BCL) | Male/Female (95% BCL) | Male/Female (95% BCL) |
| E, M + F, M (0) | 0.68 (0.57-0.82) | 1.03 (0.85-1.25) | 0.67 (0.56-0.81)b | 0.67 (0.56-0.81)b |
| F, M (0.5) | - | 1.05 (0.78-1.41) | 1.00 (0.48-2.06)d | 2.06 (1.14-3.72) |
| E, M (0.5) | - | 1.23 (0.91-1.67)2 | 3.60 (1.81-7.16)d,e | 1.11 (0.59-2.08) |
| F, M (1.5) | - | 1.11 (0.77-1.58)2 | 0.57 (0.35-0.92) | 0.68 (0.36-1.30) |
| E, M (1.5) | - | 1.58 (1.11-2.27)2,e | 1.71 (0.70-4.22) | 1.17 (0.55-2.40) |
| F, M (2) | 0.48 (0.32-0.72 | |||
| E, M (2) | 0.60 (0.38-0.93 | |||
| F, M (3) | - | 0.70 (0.45-1.11)1,2,d | 0.55 (0.21-1.42) | 0.43 (0.12-1.52) |
| E, M (3) | - | 3.11 (1.84-5.26)1,2,d,e | 3.00 (0.43-20.94) | 0.12 (0.02-0.77) |
| F, M (4) | 0.70 (0.28-1.78 | |||
| E, M (4) | 1.50 (0.63-3.57 | |||
| F, M (8) | 7 females1,2 | |||
| E, M (8) | 2.50 (0.56-11.21,2 | |||
| F, M (16) | 0.12 (0.02-0.771 | |||
| E, M (16) | 1.57 (0.63-3.931 | |||
| Totals: | ||||
| F, M >= 0.5 m | 0.44 (0.31-0.63)d,e | 0.98 (0.80-1.21)d | 0.65 (0.45-0.95)d | 1.13 (0.76-1.69)e |
| E, M >= 0.5 m | 0.85 (0.61-1.21)d | 1.59 (1.29-1.97)d,e | 0.92 (0.58-1.45) | 1.13 (0.76-1.69)e |
| Both >= 0.5 m | 0.61 (0.48-0.78)d,e | 1.25 (1.08-1.44) | 1.10 (0.83-1.47)e | 1.04 (0.77-1.40)e |
 Temnochila chlorodia (Mannerheim), a predator of D. brevicomis and
known to be attracted to E (Bedard et al., 1969), was caught in 1978 only on
the traps containing E, M (11 beetles) except for one caught on F, M at the
2-m spacing. In 1985 on the horizontal traps, T. chlorodia were caught only on
the E, M-containing traps (16 males, 6 females) except for two males and one
female on the F, M traps at 1.5 m. The vertical traps caught these predatory
beetles again only on traps with E, M (18 males, 6 females) except for one male
and two females on F, M at 1.5 m and one female on F, M at 0.5 m.
Temnochila chlorodia (Mannerheim), a predator of D. brevicomis and
known to be attracted to E (Bedard et al., 1969), was caught in 1978 only on
the traps containing E, M (11 beetles) except for one caught on F, M at the
2-m spacing. In 1985 on the horizontal traps, T. chlorodia were caught only on
the E, M-containing traps (16 males, 6 females) except for two males and one
female on the F, M traps at 1.5 m. The vertical traps caught these predatory
beetles again only on traps with E, M (18 males, 6 females) except for one male
and two females on F, M at 1.5 m and one female on F, M at 0.5 m.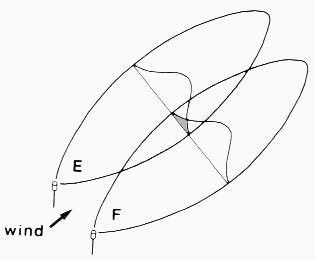
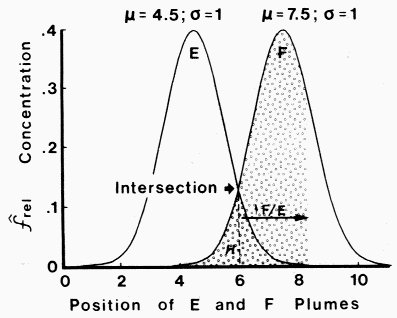
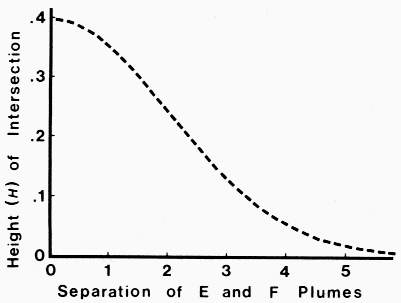
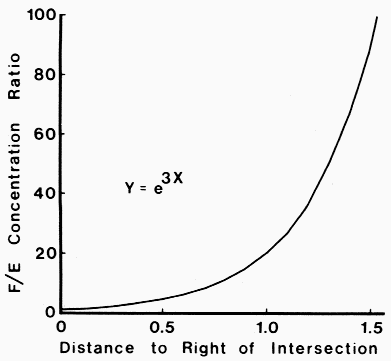
|
JOHN A. BYERS Department of Entomological Sciences, University of California at Berkeley, Berkeley, CA 94720 Present address: 
|
|---|