 Sticky-traps on 12-m high pole (traps spaced every 1.2 m) baited with pheromone. Note second pole about 100 m away in the right-center
of picture near tree line.
Sticky-traps on 12-m high pole (traps spaced every 1.2 m) baited with pheromone. Note second pole about 100 m away in the right-center
of picture near tree line. Sticy-traps on 12-m high pole (traps spaced every 1.2 m).
Sticy-traps on 12-m high pole (traps spaced every 1.2 m).
| Catch = 2 x radius x time x speed x density |
|---|
| Density = catch/(2 x radius x time x speed) |
|---|
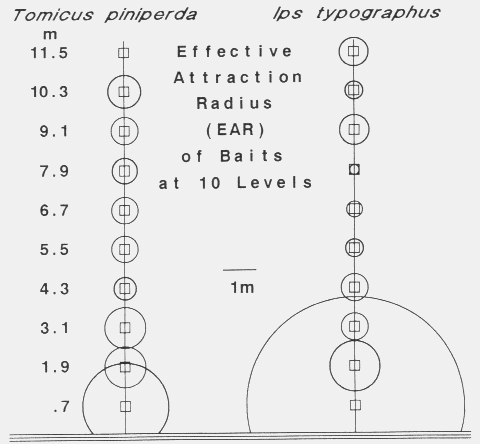
| EAR = (ATC x LCSAPT x PTC-1 x 3.141593-1)1/2 |
|---|
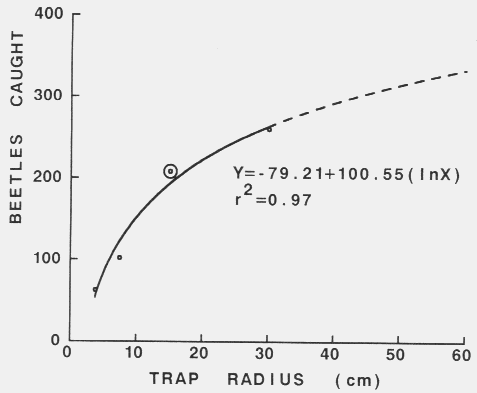
| TABLE 1. Number of bark beetles caught on passive and active sticky-screen traps, trapping periods, and wind speeds used to calculate EARs of baits and densities of flying beetlesa | |||||||||
| Beetles caught | Flying beetles/hextare estimated from passive trap poles | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Date 1984 | Passive traps | Active traps | Wind (m/sec) | Trapping duration (min) | EARb ( m ) | A | B | C | Average |
| Tomicus piniperdac | |||||||||
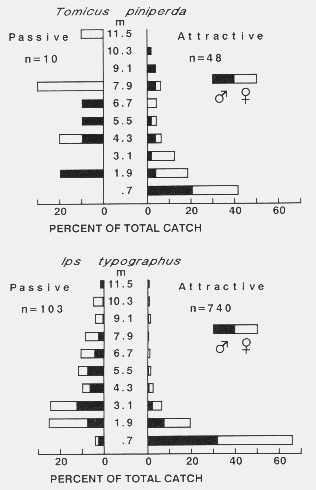
| April 15 | 7 | 27 | < 0.5 | 240 | 0.58 | 17 | 6 | 17 | 13 |
| April 21 | 3 | 19 | < 0.5 | 240 | 0.74 | 6 | 12 | 0 | 6 |
| Ips typographusd | |||||||||
| May 17 | 17 | 194 | 2.23 | 150 | 0.99 | 93 | 13 | 7 | 38 |
| May 19 | 4 | 18 | 4.00 | 160 | 0.62 | 14 | 0 | 0 | 5 |
| May 20 | 50 | 269 | 2.70 | 250 | 0.68 | 128 | 30 | 7 | 55 |
| May 21 | 15 | 44 | 3.76 | 235 | 0.41 | ( > 58)e | 30 | 8 | (> 32) |
| May 22 | 17 | 215 | 1.56 | 135 | 0.85 | (148)f | 32 | 90 | |
| TABLE 2. Densities of flying bark beetles in Scots pine forest near Angelholm, Sweden, during afternoons (1330-1730 hr) of spring days as estimated from passive sticky screens on 12-m polesa | ||||
| Flying beetles per hectare estimated from trap poles | ||||
|---|---|---|---|---|
| Species | A | B | C | Average |
| April 15, 1984 | ||||
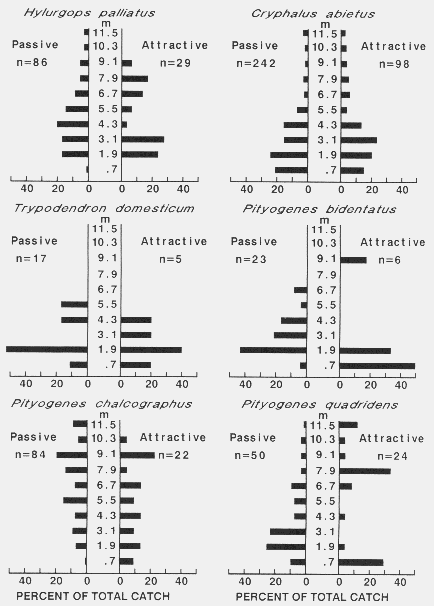
| Hylurgops palliatus | 52 | 179 | 81 | 104 |
| Trypodendron domesticum | 6 | 29 | 17 | 17 |
| April 21, 1984 | ||||
| Hylurgops palliatus | 6 | 29 | 98 | 44 |
| Trypodendron domesticum | 6 | 6 | 12 | 8 |
| May 1, 1984 | ||||
| Hylurgops palliatus | 12 | 23 | 17 | 17 |
| Trypodendron domesticum | 6 | 12 | 6 | 8 |
| Pityogenes chalcographus | 174 | 87 | 226 | 162 |
| Pityogenes quadridens | 116 | 81 | 93 | 97 |
| Pityogenes bidentatus | 52 | 75 | 6 | 44 |
| Cryphalus abietis | 307 | 729 | 365 | 467 |
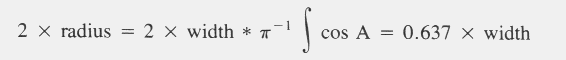
|
J. A. BYERS1,2, O. ANDERBRANT1, J. LÖFQVIST1,2 1Department of Animal Ecology, Lund University, SE-223 62 Lund, Sweden 2 Present address: 
|
|---|