1Department of Animal Ecology, Lund University, SE-223 62 Lund, Sweden
Present address:

2University College of Sundsvall/Härnösand, Box 860, SE-851 24 Sundsvall, Sweden
3Department of Organic Chemistry, Royal Institute of Technology, SE-100 44 Stockholm, Sweden
4Chemical Ecology, Göteborg University, SE-405 30 Göteborg, Sweden
 Male Pityogenes chalcographus searching for place to bore into Norway spruce. The male feeds in the phloem layer (about 2-3 mm thick)
that is under the thin outer bark (2-3 mm thick) and produces aggregation
pheromone components, chalcogran and methyl 2,4-decadienoate. Females do not produce these components.
Male Pityogenes chalcographus searching for place to bore into Norway spruce. The male feeds in the phloem layer (about 2-3 mm thick)
that is under the thin outer bark (2-3 mm thick) and produces aggregation
pheromone components, chalcogran and methyl 2,4-decadienoate. Females do not produce these components.
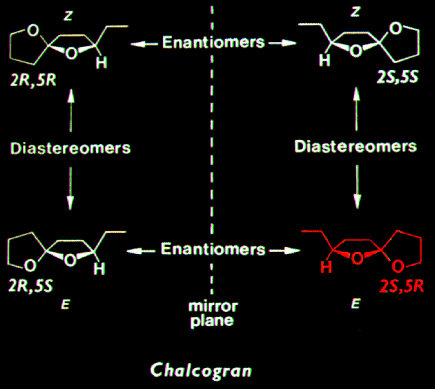
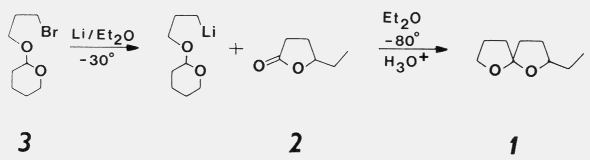
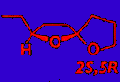 was the most active stereoisomer, while 2R,5R and 2R,5S isomers
had intermediate activities, and the 2S,5S isomer was inactive. There was no
evidence that the relatively less active stereoisomers of chalcogran inhibited
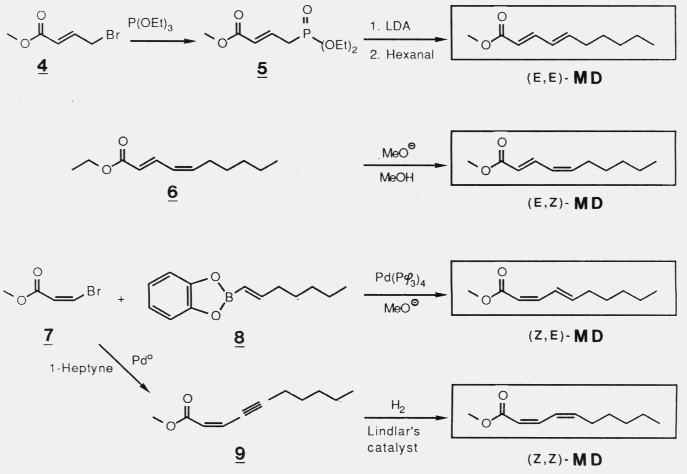
or promoted attraction to (2S,5R)-chalcogran with (E,Z)-MD. Male beetles
only produce the active E,Z isomer of MD (inactive alone) and their hindguts
contain the most active (2S,5R)- and least active (2S,5S)-chalcogran. A mixture
of all MD isomers with racemic chalcogran was not significantly different
in attractivity compared to (E,Z)-MD with racemic chalcogran, indicating
no synergistic or inhibitory effects of the inactive isomers of MD.
was the most active stereoisomer, while 2R,5R and 2R,5S isomers
had intermediate activities, and the 2S,5S isomer was inactive. There was no
evidence that the relatively less active stereoisomers of chalcogran inhibited
or promoted attraction to (2S,5R)-chalcogran with (E,Z)-MD. Male beetles
only produce the active E,Z isomer of MD (inactive alone) and their hindguts
contain the most active (2S,5R)- and least active (2S,5S)-chalcogran. A mixture
of all MD isomers with racemic chalcogran was not significantly different
in attractivity compared to (E,Z)-MD with racemic chalcogran, indicating
no synergistic or inhibitory effects of the inactive isomers of MD.