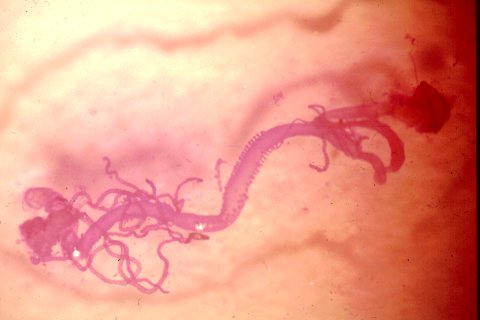
 Mid- and hindgut of Ips paraconfusus bark beetle. The rectum and last abdominal segment are at the
upper right of picture. In the center is the posterior midgut (ventriculus) with the two rows of papillae. Biosynthesis
of ipsenol and ipsdienol my occur in the anterior part of gut according to recent reports.
Mid- and hindgut of Ips paraconfusus bark beetle. The rectum and last abdominal segment are at the
upper right of picture. In the center is the posterior midgut (ventriculus) with the two rows of papillae. Biosynthesis
of ipsenol and ipsdienol my occur in the anterior part of gut according to recent reports.
| Table 1. The relationship between the amount of myrcene added to the bottle and the concentration* of myrcene in the headspace and the subsequent production of ipsenol and ipsdienol in male I. pararonfusus | |||||||||
| µ Myrcene added to aeration chamber | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 2.1 | 3.6 | 9a | 12.7 | 24.1 | 45.8 | 74 | 93 | |
| Concentration of myrcene in headspace (10-7 g/ml) | 0 | 3.4 | 6.8 | 10 | 13.5 | 24.5 | 40.3 | 57.5 | 66.1 |
| Per cent comatose | 0 | 0 | 0 | 0 | 1 | 3 | 10 | 60 | 95 |
| Amount of ipsenol produced per male (10-8 g) | 0 | 12.5 | 21.5 | 26.5 | 29.8 | 37.3 | 19.4 | 16.8 | 15.3 |
| Amount of ipsdienol produced per male (10-8 g) | 0 | 2.2 | 4.2 | 5.3 | 6.1 | 7.7 | 4.4 | 3.7 | 3.1 |
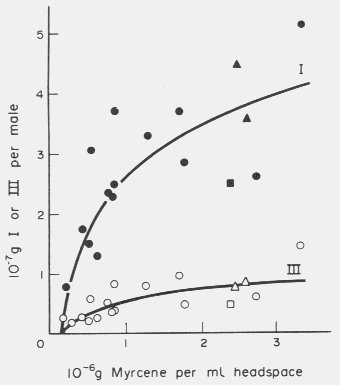 Fig. 1. I (ipsenol) and III (ipsdienol) production in male I. paraconfusus after exposure
to myrcene vapours. Solid symbols report production of ipsenol while open symbols report
production of ipsdienol.
Fig. 1. I (ipsenol) and III (ipsdienol) production in male I. paraconfusus after exposure
to myrcene vapours. Solid symbols report production of ipsenol while open symbols report
production of ipsdienol.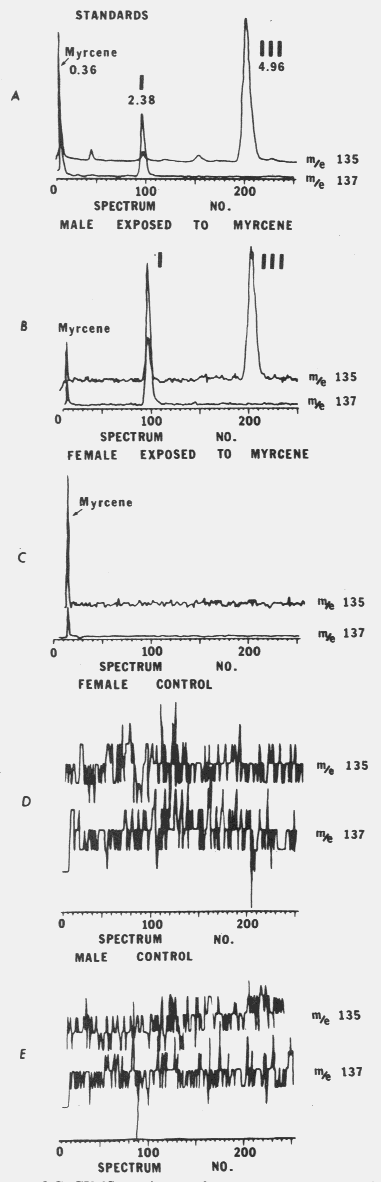
| Table 2. Response of female I. paraconfusus to pheromonal components (I = ipsenol; II = cis-verbenol; III = ipsdienol) and to gut extracts from beetles exposed to various treatments in the bioassay | |||||
| Extract | Rate of delivery per min per compound | Per cent females responding | (C.I.)* | Number of females | |
|---|---|---|---|---|---|
| Assay for presence of I and III together (20 April 1976) | |||||
| Female guts (no myrcene) | 0.09 gut equivalents | 2a | (0 - 8) | 60 | |
| Male guts (no myrcene) | 0.09 gut equivalents | 3a | (1 - 12) | 60 | |
| Female guts (myrcene)¥ | 0.09 gut equivalents§ | 2a | (0 - 8) | 90 | |
| Male guts (myrcene)@ | 0.09 gut equivalents¤ | 59d | (48 - 69) | 90 | |
| I + III | 8.9 x 10-10 g | 23b | (11 - 38) | 40 | |
| I + III | 8.9 x 10-9 g | 33bc | (17 - 52) | 30 | |
| I + III | 8.9 x 10-8 g | 57cd | (38 - 75) | 30 | |
| I + III | 8.9 x 10-7 g | 70d | (51 - 85) | 30 | |
| Assay for presence of III only (29 July 1976) | |||||
| I | 8.9 x 10-9 g | 13a | ( 4 - 47 ) | 40 | |
| I + III | 8.9 x 10-10 g + 1.8 x 10-10 g | 50b | (32 - 69) | 30 | |
| I + Female guts (myrcene)¶ | 8.9 x 10-9 g + 0.8 gut equiv.§ | 7a | (5 - 19) | 30 | |
| I + Female guts (myrcene)¥ | 8.9 x 10-9 g + 0.8 gut equiv.§ | 10a | (2 - 27) | 30 | |
| Assay for presence of I only (7 November 1977) | |||||
| I | 8.9 x 10-10 g | 10a | (2 - 26) | 30 | |
| II + III | 8.9 x 10-10 g | 7a | (0 - 22) | 30 | |
| I + II + III | 8.9 x 10-10 g | 43b | (25 - 62) | 40 | |
| II + III + Female guts (myrcene)@ | 8.9 x 10-9 g + 0.8 gut equiv. | 10a | (2 - 24) | 40 | |
| II + III + Female guts (myrcene)¥ | 8.9 x 10-9 g + 0.8 gut equiv.§ | 0a | (0 - 12) | 30 | |