Byers, J.A. 1991. Pheromones and chemical ecology of locusts. Biological Reviews. 66:347-378.  pdf
pdf
By JOHN A. BYERS
Department of Animal Ecology, Lund University, S-223 62 Lund, Sweden
Present address: 
CONTENTS
I. Introduction
 (1) Host plants and feeding
(1) Host plants and feeding
 (2) Life history and pheromones
(2) Life history and pheromones
 (3) Principles of isolation of pheromone components
(3) Principles of isolation of pheromone components
II. Gregarization pheromone
 (1) Physiological effects
(1) Physiological effects
 (a) Isolation of pheromone
(a) Isolation of pheromone
 (2) Behavioural effects
(2) Behavioural effects
 (a) Isolation of pheromone
(a) Isolation of pheromone
 (3) Source and biosynthesis
(3) Source and biosynthesis
 (4) Reception
(4) Reception
 (5) Ecology
(5) Ecology
III. Adult maturation pheromone
 (1) Vibration reaction
(1) Vibration reaction
 (2) Reception
(2) Reception
 (3) Source and physiological regulation
(3) Source and physiological regulation
 (4) Ecology
(4) Ecology
IV. Oviposition-stimulating pheromone
V. Oviposition-aggregating pheromone
 (1) Reception and Source
(1) Reception and Source
 (2) Ecology
(2) Ecology
VI. Other possible pheromones and semiochemicals
VII. Control
VIII. Conclusions
IX. Summary
X. Acknowledgements
XI. References
I. INTRODUCTION
Of the several species of desert and migratory locusts, two species cause most of the economic losses and have
consequently received much attention, the desert locust, Schistocerca gregaria (Forskål), and the African migratory
locust, Locusta migratoria L. (Orthoptera: Acrididae). These two species have served as model experimental animals
for a great number of studies. Over 4500 research papers have dealt with locusts since 1970 according to Biological
Abstracts (BIOSIS). A personal computer search of the titles and key word listings from BIOSIS showed that 69% of the
papers concerned these two species. Most papers are physiological and involve laboratory experiments, while the fewer
behavioural and ecological studies are also mostly in the laboratory using insects reared in cultures. Relatively few
behavioural and ecological studies in nature have been undertaken in recent times (last 20 years). Thus it is often difficult to
assess the more numerous laboratory studies in terms of how they relate to properties of natural populations. Earlier
investigations on natural population ecology, however, are reported in the Anti-Locust Bulletin, (Johnston & Buxton, 1949;
Ellis, 1951; Joyce, 1952; Popov, 1953, 1958; Dirsh, 1953; Guichard, 1955; Davey & Johnston, 1956; Ellis & Ashall, 1957;
Stower, Popov & Greathead, 1958; Ashall & Ellis 1961).
This review will attempt to cover in some detail the evidence for the various pheromones hypothesized for locusts. The
physiological, morphological, and behavioural aspects of pheromone studies will be integrated with concepts of ecology with
the idea of developing control strategies. Aspects of chemical ecology related to pheromones, such as feeding stimulants and
deterrents will be covered briefly as well as some other topics for the sake of clarity and interest. The earlier work on locust
pheromones will be shown to be incomplete and sometimes contradictory. Thus, it is not often possible to compare the
knowledge of locust pheromones to recent findings in other groups of insects.
A recent review of pheromones and phase transformation in locusts by Loher (1990) also points out certain problems with
earlier work although different aspects are stressed compared to those here. Grasshopper chemical communication, including
locusts, are also discussed by Whitman (1990). For comparisons to other insect systems, the reader is referred to recent
reviews on the pheromone systems of bark beetles (Scolytidae: Coleoptera), moths (Lepidoptera) and social insects (Byers,
1989; Baker, 1989; Ali & Morgan, 1990).
(1) Host plants and feeding
Schistocerca gregaria has been termed polyphagous (Evans & Bell, 1979) and its food preferences have been
investigated by several authors (Mann & Burns, 1927; Bhatia, 1940; Husain, Mathur & Roonwal, 1946; Alam 1952; Pradhan,
Jotwani & Rai, 1962; Rao & Mehrotra, 1977; Singh & Pant, 1980). Of 198 plants from a variety of families screened by
Husain et al. (1946), nine plants were not eaten at all, 29 were eaten with reluctance while 160 plants were eaten readily.
Locusta migratoria, in contrast, has been termed a 'specialist' feeder (Evans & Bell, 1979) or oligophagous (Simpson,
Simmonds & Blaney, 1988) because individuals eat only the grass family (Poaceae, formerly Gramineae), although a variety
of species.
In Mali, Ohabuike (1979) found no relationship between the abundance of grass species found in faecal pellets of
Locusta migratoria to the abundance of these grasses found living in the field, indicating that certain grass species
were preferentially eaten. Preference for certain species also changed during the season depending on water content. Thus
during the dry periods perennial grasses of higher water content were chosen over dryer annual grasses that formerly were
preferred in the rainy or flood-retreating seasons. However, practically all species were eaten to some extent, even those
suspected of containing feeding deterrents. These latter species retarded locust growth but were preferred during the dry
season due to their higher water content.
(2) Life history and pheromones
In the core outbreak area in Mali where the flood plains of the Niger and Bani rivers converge, Locusta migratoria
usually has four generations per year (Ohabuike, 1979). Depending on periodic rainfall and grass growth, the population of
L. migratoria may increase in density resulting in behavioural, physiological, and morphological changes as the young
hoppers develop to adults. Similar development of locust plagues for Schistocerca gregaria have been reported
(Bennett, 1975; Waloff & Green, 1975). These changes induced by favorable climatic conditions and resurgent food supplies
were first documented by Uvarov (1921) in his theory of periodicity of locust migrations.
Under endemic conditions the 'solitary phase' or form predominates. In Locusta migratoria, these young hoppers are
green and remain so as adults, but under epidemic high-density conditions individuals show various degrees of the
'gregarious phase', where the hoppers darken and as adults are highly pigmented with melanin (Nolte, 1977). Other
morphological features (morphometric ratios) change such as the ratios of the hind femur length to the head capsule width or
pronotal length to width. The same general phenomenon occurs in Schistocerca gregaria but the colour and
morphological changes are different (Dirsh, 1953; Nolte, 1976; Gillett, 1975b). Not only does the colour darken but gregarious
behaviour develops and large increases in flight capacity are evident (Nolte, 1977; Michel, 1980). The transformation of
phases can be in either direction during development, depending on the local population density, but is usually toward the
gregarious form in preparation for migration. A pheromone, called the 'gregarization pheromone' (Fig. 1) and possibly other
pheromones are involved in phase transformation and social cohesion (Nolte, May & Thomas, 1970; Nolte, Eggers & May,
1973; Gillett 1975a; Fuzeau-Braesch et al., 1988).

Fig. 1. Experimental evidence of pheromonal effects on various stages of locust (Locusta migratoria, L.m., and
Schistocerca gregaria, S.g.). Dashed lines and arrows indicate the relationship between the stages producing
pheromone and those affected by pheromone. See text for more details.
The life cycle of both species begins with the hatching of eggs, laid in groups of about 50 per egg pod in the sand (Harjai &
Sikka, 1970). The hoppers undergo six or seven instars (seven if they remain solitary, Gillett, 1975b) before becoming adult.
In Locusta migratoria, adults attain sexual maturity within a week or so while Schistocerca gregaria requires
from two weeks to one month depending on the presence of other locusts (Norris, 1964). An adult maturation pheromone
(Fig. 1), produced by males of S. gregaria, has been shown to promote the sexual development of both sexes and
synchronize the maturation of the group (Norris, 1954; Loher, 1960). An anti-maturation pheromone produced by young
nymphs may retard the maturation of adults (Norris, 1954, 1962). After maturing, females of S. gregaria that have
mated develop mature eggs, due in part to a male pheromone transferred during copulation (Fig. 1, Lange & Loughton,
1985), and begin seeking suitable places in the sand to oviposit. In L. migratoria a 'long-range' (many decimeters)
pheromone seems to attract females to an area previously used by other females for oviposition (Fig. 1, Lauga & Hatte,
1977, 1978). Another pheromone attracts female S. gregaria over a few centimeters to areas with locusts where
oviposition is then stimulated (Fig. 1, Norris, 1963, 1970). Each of the above pheromones (depicted in Fig. 1) will be
discussed in the remainder of the paper in terms of (1) the evidence for the pheromone, (2) isolation and source of
pheromone, (3) identification of chemical structure, (4) sensory perception, and (5) ecological and evolutionary aspects.
Before beginning a discussion of the various pheromones, it is appropriate to explain the chemical and behavioural methods
for isolation and identification of pheromones. A firm understanding of the requisite pheromone components of a particular
species is crucial before one can proceed with other basic studies or with development of control methods using
pheromones.
(3) Principles of isolation of pheromone components
As mentioned above, most locust research, including that with pheromones, has been undertaken in the laboratory with
artificially reared insects. This situation is ill-advised if basic studies of behaviour and ecology, with regard to modern
principles, have not been well investigated in the field. The first step in the study of a pheromone is the observation of the
natural phenomenon. Once this knowledge is obtained it is possible to develop relevant bioassays in the laboratory as part of
the methodology required for pheromone isolation. Some insight into the source of the pheromone, how and when it is
transferred or released to other individuals, and the conditions necessary for response must be gotten in order to construct a
bioassay. This is often difficult since several factors may vary simultaneously. Therefore, without a reliable bioassay that is
relevant to the natural behaviour there is no point in continuing with the isolation work.
Once a suitable bioassay is developed, and it can be shown that extracts of the emitting insect have specific effects on
receiving insects, then chromatographic separation of the chemical blend can proceed. At every stage of further separation of
the pheromone extract into various chemicals, bioassays are used to locate the activity among the fractions. Unfortunately,
no studies of the various locust pheromones have advanced beyond the initial separation stage and bioassay of individual
fractions or compounds (Nolte et al., 1973; Nolte, 1976), or they have assayed compounds based on their dominance in an
extract (Fuzeau-Braesch et al., 1988). Synergism, where several compounds often widely separated during chromatography
must be presented together to elicit maximal response, is usually the case in most insects (Silverstein, 1981; Byers et al.,
1990). Tests for synergism among compounds have rarely been done with locust pheromones and then only with a mixture
of all compounds identified in the extract (Fuzeau-Braesch et al., 1988).
During the isolation, extracts as well as purified compounds must be released at known rates in the behavioural bioassay (or
in electrophysiological recordings of the antenna or sensillum). Otherwise it is possible that biologically insignificant
compounds would seem active because of their release at unusually high concentrations compared to biologically significant
compounds released at relatively low rates. Rigorous quantification of these semiochemical rates is lacking in locust studies
and is a shortcoming of pheromone studies in general (Byers, 1988). Ultimately, the compounds indicated from the laboratory
investigations must be tested in the field at release rates comparable to those expected from insects in nature. A recent
technique called 'diffusion-dilution', where release rate is predicted from the mole percentage dilution of the component in
solvent and the dimensions of the releasing tube, should make it easier to obtain precise release rates of volatile pheromone
components and semiochemicals in both the laboratory and field (Byers, 1988).
II. GREGARIZATION PHEROMONE
There is little doubt that a gregarization pheromone exists in Schistocerca gregaria (and another in Locusta
migratoria) that mediates phase transformation toward the gregarious form and elicits aggregation behaviour. However,
whether one compound (locustol) or several participate in one or more of the various changes, which include colour,
morphometric ratios (elytron/femur lengths, femur length/head width), chiasma formation rate during meiosis, development
time and molting synchronization, and cohesive behaviour, is poorly understood. Solutions to these questions have been
hindered by (1) the relatively slow changes induced by pheromone (up to several weeks), (2) by the possible application of
inappropriate bioassays, and (3) by few, if any, attempts to confirm effects in the field.
(1) Physiological effects
Nolte (1963) observed that after third instar 'crowded' hoppers were isolated individually in another room they gradually lost
their black pigment at each moult and became green or sandy coloured. Experiments that transplanted nymphs from either
isolated or crowded environments indicated the presence of a pheromone in the air surrounding crowded hoppers. The
pheromone induced both melanization and to some extent the morphometric ratios characteristic of the gregarious phase. A
few years later Nolte (1967, 1968, 1969) reported that spermatocytes of Schistocerca gregaria exhibited a higher
frequency of chiasmata (crossover points of DNA between non-sister chromatids of a chromosome during the diplotene stage
of meiosis I) in only those individuals exposed to crowded conditions (pheromone present) as well as adults in the gregarious
phase from an outbreak in the field. These crowded locust nymphs remained gregarious in form (dark colour) when compared
with isolated individuals that became more solitary in form and lighter in colour with subsequent molting.
Chiasma frequency increases have been suggested to be the most sensitive and reliable indicator of pheromone-induced
phase transformation to the gregarious form (Nolte et al., 1970; Nolte, 1973; Nolte, 1976). However, Dearn (1974a, b)
criticized earlier work on chiasma frequencies because of small sample size and more importantly he found no significant
differences between solitary and gregarious locusts of Schistocerca gregaria and Locusta migratoria in terms of
chiasmata. Nolte (1976) countered with the hypothesis that Dearn's use of white paper during rearing caused homochromy
so that solitaries were pale creamy to off-white colour instead of the usual green. The experiments showed individuals reared
on white paper had chiasma frequencies that increased up to 29% compared with green controls (Nolte, 1976) and these
magnitudes are comparable to gregarious phase frequencies for chiasmata (Nolte, 1967, 1973). He concluded that his
method when employed properly was valid for discriminating the solitary from the gregarious phase and in quantification of
the effects of the gregarization pheromone. However, Nolte's method and results have not been confirmed by other workers.
Also it seems possible that chiasma frequency changes are associated with colour changes and not directly with pheromone
dosage so this method may not be the most reliable and relevant bioassay for the pheromone. This hypothesis is
strengthened by results with albino L. migratoria in which neither crowding nor "synthetic pheromone" increased the
chiasma frequencies (Nolte, 1976).
Gillett (1968) found evidence for the gregarization pheromone, or other airborne pheromone, in Schistocerca gregaria
which had a behavioural effect, causing solitary hoppers to show grouping behaviour after exposure to air from crowded
hoppers, or to lose such behaviour if reared in 'clean' air. The term 'gregarization pheromone' was then introduced by Nolte
et al. (1970) to include all of the above effects. However, the 'pheromone' has not yet been shown to have a common
chemical structure in both locust species and in fact appears to have different behavioural and physiological effects (Nolte,
1973; Gillett, 1983).
Still another effect of the gregarization pheromone was added by Gillett (1975b). She found that isolated hoppers of
Schistocerca gregaria reared in 'clean' air often undergo an extra instar compared to hoppers isolated but exposed to
pheromone in the air from crowded hoppers. The development time to reach the fifth instar was significantly less for crowded,
pheromone-exposed hoppers (21 days) than for isolated hoppers exposed to pheromone laden air (29 days), while still more
for isolated hoppers in 'clean' air (37 days). However, the data for crowded hoppers is not comparable to the latter two values
since the crowded individuals were free to approach the light bulb and thus optimize their temperature regime. This freedom
of movement may also have resulted in the "molting synchronization" of crowded hoppers compared with isolated hoppers.
However, the only valid comparison is between the isolated hoppers exposed or not to pheromone. The crowded hoppers
had different possibilities to regulate their temperature while field collected F1 solitaria were of a different genetic
line and history. The valid comparison did show that pheromone-exposed hoppers were more synchronized (lower variance in
length of fifth instar) than control, isolated hoppers (Gillett, 1975b).
In Locusta migratoria it was also found that pheromone from hoppers, but not adults, shortens the duration of the fifth
instar and thus synchronizes molting (Nolte, 1976). Loher (1960) first reported that the epidermis of mature males of the
gregarious form of Schistocerca gregaria is thicker and contains vacuolated cells that appear to produce the
maturation pheromone. This finding was confirmed by Thomas (1970) and Strong (1970, 1971). The maturation pheromone
will be discussed later but it appears to shorten the maturation period of adults and thus synchronizes development of
locusts. Other phase characteristics in S. gregaria were little affected by pheromone treatment such as number of eye
stripes and morphometric ratios, although colour was affected as expected (Gillett, 1975b). Nolte et al. (1970) also found little
effect of pheromone on morphometric ratios in L. migratoria, although in other studies they found stronger
morphometric effects (Gillett, 1968; Nolte et al., 1973; Nolte, 1976). The problem with these studies is that the concentration
or release rate of pheromone is not known so quantitative comparisons between treatments in various experiments are not
possible.
(a) Isolation of pheromone
The first attempt to isolate a gregarization pheromone used solvents of either risella oil or dimethyl sulphoxide to extract the
air from a locust (Locusta migratoria) breeding room (Nolte 1968). Hoppers that had been reared crowded until the
third instar were isolated and exposed to extracts, after evaporation of solvent, during the fourth and fifth instars. High
chiasma frequencies in young adults compared to controls were found as well as a retention of the dark coloration
(subjectively judged, Nolte et al., 1970). Oil extracts of the head, thorax and abdomen and portions of the alimentary tract
were tested similarly on isolated hoppers and revealed that the highest chiasma frequencies were associated with extracts of
the crop. Further experiments indicated that the pheromone is excreted with the faeces in both sexes, regardless of whether
the hoppers are crowded or not. More pheromone is released by crowded hoppers simply due to the higher numbers and
quantity of faeces. In contrast, adult locusts or their faeces did not affect phase transformation (or chiasma frequencies)
(Nolte et al., 1970, 1973; Nolte, 1976). Gillett & Phillips (1977) agree that nymphal faeces (fourth and fifth instar) of
Schistocerca gregaria are also active in promoting social aggregation and dark coloration in hoppers while adult
faeces appear to have no effect.
With the advent of a supposedly reliable bioassay and knowledge of the source of pheromone it was possible to attempt
isolation of the chemical components. Nolte et al. (1973) steam distilled 1 kg of faeces from crowded Locusta
migratoria hoppers and then extracted the distillate with pentane. The pentane extract when concentrated in vacuo
and subjected to silica gel thin layer chromatography and gas chromatography (GC) showed two major and several minor
constituents. One major component was guaiacol of which the o- and p-isomers (Fig.1) were most active in the
chiasma frequency bioassay, although which of these isomers, or the m-isomer, were present was not determined.
The second major component was identified by gas chromatography-mass spectrometry (GC-MS) and nuclear magnetic
resonance spectrometry, synthesis of the indicated possible structures, and comparison by GC-MS of the synthetic with the
biological substance. The compound was called "locustol" (2-methoxy-5-ethylphenol, also called 5-ethylguaiacol, Fig. 2), and
it was the most active of any tested substance in the chiasma bioassay. Locustol at an unknown concentration when
volatilized in cages with isolated hoppers was able to prolong the retention of dark pigmentation in formerly crowded hoppers
by about 80% (as judged subjectively) compared to controls. Morphometric ratios were slightly affected by locustol but not
significantly by isomers of guaiacol. An immediate effect of locustol but not guaiacol (o-isomer, Fig. 2) on the marching
behaviour and aggregation near a source of release was also noted (Nolte et al., 1973). They state that locustol "comes
closest" to being the gregarization pheromone. They caution that there are "several other compounds each possessing
varying degrees of activity, affecting one or other of the gregarization traits."
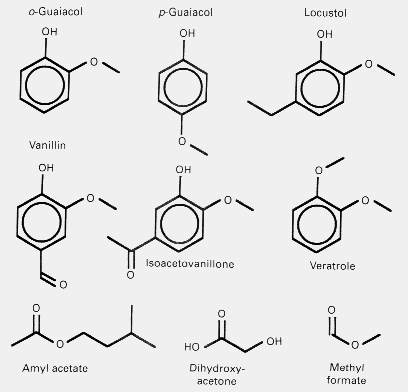
Fig. 2. Potential pheromone candidates affecting melanization, chiasma frequencies, and social grouping behaviour in
locusts.
Locusta migratoria nymphs were subjected to hopper faeces from each of four locust species to ascertain the effects
on melanin retention and chiasma frequency (when adult). Faeces from the red locust, Nomadacris septemfasciata,
and Australian locust, Chortoicetes terminifera, had no effect while faeces from Schistocerca gregaria and the
brown locust, Locustana pardalina, both reduced the loss of black pigment and increased the chiasma frequency
(Nolte, 1973). No effects of crowding the grasshopper, Paracinema tricolor, were observed on the colour or the
chiasma frequency, indicating no pheromonal effects. Ba-Angood (1976) reared S. gregaria individuals alone, crowded
in groups of 10 and individually with 9 grasshoppers (mixture of Oedalens spp., Aiolopus spp. and
Kraussaria spp.). There was no gregarizing effect of the grasshoppers on morphometric ratios or eye stripes of S.
gregaria compared to the solitaries while the crowded S. gregaria showed phase transformation to gregaria. These
results indicate that the gregarization pheromone is general only to certain of the locust species.
Nolte (1976) proceeded to further purify locustol which he admitted was previously "synthesized by the old method," and
"this was impure." He also steam distilled and extracted with diethyl ether 2 kg faeces of hoppers and of adults as well as a
smaller amount of grasshopper faeces (probably P. tricolor). The infrared spectra of hopper faeces extract showed a
strong aromatic peak while adult or grasshopper faeces extracts had no such peak, consistent with the aromatic structure of
locustol and the bioassay activity of hopper faeces. However, GC was unable to confirm the presence of locustol, although
Nolte (1976) unfortunately looked "two months after the bioassay so that any pheromone present could have sublimed." This
seems unlikely, assuming the ether extract was intact, since locustol is much less volatile than ether and thus would have
tended to concentrate.
In any case, the hopper faeces extracts of Locusta migratoria were active in increasing the chiasma frequency, the
retention of melanin (subjective), and the F/C (ratio of hind femur to headwidth), and synchronized molting by shortening the
fifth instar period (Nolte, 1976). Although locustol was most active in increasing chiasma frequencies there were several other
analogues, including guaiacol isomers, vanillin, as well as dihydroxyacetone (Fig. 2) which elicited significant increases in
chiasma frequencies. However, air from crowded hoppers was most active in reducing or slowing melanin loss, while hopper
faeces extracts were significantly less active. Little effect could be observed from locustol alone and this was not significantly
different from isoacetovanillone, guaiacol, amylacetate (Fig. 2), or adult faeces extract. The F/C ratios were most affected by
air from crowded hoppers and by amylacetate, and less so but significantly by hopper faeces extracts and by locustol.
Methylformate (Fig. 2) stimulates melanin production in solitaries to the level of that of crowded controls (Nolte et al., 1973;
Nolte, 1976). Gillett (1983) tested locustol as well as extracts of faeces of either L. migratoria or Schistocerca
gregaria. In this case only the S. gregaria faeces were active in affecting melanization and grouping behaviour.
Thus, while S. gregaria faeces may effect L. migratoria phase transformation the converse does not seem true
(Nolte, 1973; Gillett, 1983).
The apparently pheromonal effects caused by solvent chemicals again indicate the need for more reliable, specific, and
relevant bioassays for further progress in elucidating the components of the gregarization pheromone. The fact that locustol
was given at 0.1%, and guaiacol among others at 0.5%, while 0.25% locustol and 1% guaiacol are "lethal" seriously
jeopardizes the validity of the results (Nolte, 1976). A re-isolation of the gregarization pheromone is necessary using modern
analytical methods and the subtractive-combination bioassay (Byers et al., 1990). The latter method allows isolation of
synergistic components with a minimum number of tests (compared to additive methods) and is thus optimally suited to the
problem.
(2) Behavioural effects
In addition to the 'primer' effects of the gregarization pheromone there are apparently immediate 'releaser' effects on the
behaviour. The effect of locustol on marching behaviour has already been mentioned. Gillett and co-workers have studied the
immediate and longer-term behavioural effects of the gregarization pheromone (Gillett, 1968, 1975a, 1988; Gillett, Packham,
& Papworth, 1976; Gillett & Phillips, 1977). Gillett (1975a) placed ten second instar nymphs or ten immature adults, reared
either isolated or crowded, in a 90-cm diameter arena. At intervals of 15 min. for adults or 30 min. for nymphs, the locusts
were disturbed and then allowed to resettle during the next interval whereupon their grouping positions were noted. It was
found that significantly more grouping behaviour (number of groups of 3 individuals or more) was shown by the crowded
nymphs or adults than the respective controls that had been reared isolated (no pheromone). However, in several
experiments the rates of grouping behaviour, touching, and numbers at arena edges were sometimes inversely correlated
which is not easily explained. One mathematical problem with this method may be that strong grouping behaviour would tend
to include larger groups and lower the number of possible groups of three or more. When locust nymphs or adults were
observed for two hours they tended to 'learn to group' (i.e. increase grouping behaviour), regardless of their past exposure to
pheromone, except for the crowded nymphs which showed a high level of grouping throughout. It is not known if this is a
phenomenon of social learning or an immediate effect of the pheromone.
Gillett et al. (1976) tried another bioassay in which individual nymphs or adults of Schistocerca gregaria were
attracted down an 8 x 64 cm walkway to a light source. A porous floor of about 6 cm length was used to release volatiles
from live locusts or molt skins, while aqueous or chloroform/methanol extracts of these were evaporated on filter paper over
the porous floor. The ratios of the time to traverse the walkway to the time spent over the odor source (floor or paper) were
determined. A control was carried out on the same day as the respective treatment (it is not clear if the same or different
locusts were used). The results showed that adults were slowed in their progress to the light (arrested) only by either adult
faeces smeared on the filter paper or extracts of adult cuticle (which may have contained sex-maturation pheromone).
Nymphs were arrested by nymphal faeces on filter paper or living immature adults. In contrast, they were repelled by
aqueous or solvent extracts of nymphal faeces, as well as by living nymphs. These conflicting results are difficult to explain.
Both adults and nymphs appeared unresponsive to solvent extracts from adults or molt skins. These results are further
confounded by the fact that the above time ratios for the controls in the various experiments with nymphs varied from 4.26 to
9.34, thus widely overlapping the differences between a specific treatment ratio and its control. With such wide variation
possible between controls (and treatments) it seems the bioassay was inconsistent.
The possibility of an anti-gregarization pheromone or 'solitarizing' pheromone was first proposed by Gillett & Phillips (1977).
They based their hypothesis on the finding that isolated nymphs treated with adult faeces were even less 'gregarious' in
behaviour and colour than untreated isolated nymphs when compared with crowded controls, although the differences
between the isolated, treated and untreated, nymphs were not statistically significant. This evidence, admittedly weak, was
examined again by Gillett (1983). She reared first instar nymphs crowded and then separated them under various treatments
until mid second instar when their social behaviour was appraised. The grouping behaviour of nymphs with adult faeces was
much less than nymphs with nymphal faeces and not significantly different from full isolated nymphs. This indicates adult
faeces are ineffective in gregarizing nymphs but does not provide evidence for a solitarizing pheromone. In another
experiment (III), two nymphs were reared per jar with or without adult faeces added, but no effect on grouping behaviour
could be observed and the behaviour was not different from crowded controls. Therefore, there is no evidence to support her
conclusion that there is "evidence of a previously proposed (Gillett & Phillips, 1977) stimulus in the faeces of locusts which
modifies the phase polymorphism: a solitarizing stimulus produced by crowd-reared adults."
Gillett (1988) ignores the 'solitarization' pheromone in her recent work entitled "Solitarization in the desert locust . . ." In this
paper the gregarization pheromone seems to have a greater effect on the loss of gregarious behaviour than on its
development. Gregarization and the willingness to form groups take place rapidly within hours while the loss of grouping
behaviour when isolated occurs gradually over about 10 days. Gillett (1983) found no effect of locustol in hexane on filter
paper on the grouping behaviour and coloration of second instar Schistocerca gregaria, while an effect was found for
exposure to faeces. These results do not support locustol as the gregarization pheromone, although insufficient release or
exposure time could account for the inactivity.
(a) Isolation of pheromone
Fuzeau-Braesch et al. (1988) considered that isolation of the gregarization pheromone from air surrounding crowded locusts
(and faeces) would be more appropriate than isolation from faeces since locusts were actually responding to volatiles. The
relative proportions of candidate pheromone components could be quantified without the contamination from inappropriate
non-volatile constituents in the faeces. Also, no assumptions as to the source of pheromone, whether faeces or integument,
would be needed. Their collection of volatiles was inefficient, however, since they were collected in condensed water vapour
in an ice bath. It can be assumed that nonpolar and volatile compounds would be incompletely collected. They did collect
phenol, guaiacol and veratrole (Fig. 2) from both Locusta migratoria and Schistocerca gregaria so it is likely
that locustol also should have been collected. However, they report that locustol could not be identified by GC-MS. They
wonder if locustol could exist only in faeces or be hydrolyzed from a precursor through extraction by steam with the methods
used by Nolte et al. (1973).
Fuzeau-Braesch et al. (1988) conclude that phenol or guaiacol (isomers not determined) alone or in mixture with veratrole
act as a 'cohesion pheromone'. Thus these compounds mimic, at least partly, the behavioural grouping effect of the
gregarization pheromone. However, there are several problems with their bioassay as well as conflicts with earlier work. This
work has shown consistently that adults are not effective in causing gregarization while only nymphs are bioactive (Nolte et
al., 1970, 1973; Nolte, 1976; Gillett & Phillips, 1977; Gillett, 1983). On the other hand, Fuzeau-Braesch et al. (1988) report
that in Schistocerca gregaria "where all ages provide enough substance for easy calculation" it seems that young
immature adults have a lower concentration of volatile products. Since they inspected fifth instar nymphs as well as
copulating (mature) adults and egg-laying adults these findings contradict the earlier work.
Fuzeau-Braesch et al. (1988) used a bioassay that counted the number of locusts clumping in each of 4 arms or center of a
cross-shaped arena. Phenol, guaiacol and veratrole (Fig. 2) were released from one arm (#4) and air flowed from arms 2, 3
and 4 to 1 where locusts were released. The first problem is the use of Chi square to show a difference in distribution among
arms between treatment and control. Since there is little reason to suppose that a pheromone would cause a different
distribution among the arms but only in clumping in the center (as they hypothesize) it is probably wrong to make such a
comparison. For example, phenol caused 42 of 200 to group in the middle compared to 28 for the control (P< 0.01). The
supposedly significant result is due to a comparison of all four arms and center, while a comparison of just the center shows
no significant difference (P=0.07). According to Fuzeau-Braesch et al. (1988), of their 13 tests (Tables 4 and 5) 12 are
significant, but if one compares only the center areas then 9 of the tests are significantly different. Unless an error in printing
the value for guaiacol occurred, the compound appears surprisingly to have induced significantly less grouping than the
control in Locusta migratoria. Also the controls for L. migratoria varied from 16 to 52 in the center while
treatments varied from 34 to 57. Unfortunately, locustol was not tested. Another problem is that these compounds were
chosen based on their dominance in the extract. Minor components and one unidentified major component were not tested
so their importance could have been missed. Neither additive- nor subtractive-combination bioassays were attempted that
could have rigorously tested for synergists (Byers et al., 1990). In spite of the statistical criticism for individual experiments, it
does seem that an immediate pheromone effect on grouping can be shown in their bioassay.
(3) Source and biosynthesis
Locustol is postulated to be synthesized from guaiacol, a degradation product of lignin that is ingested in grass or shrubs
(Nolte et al., 1973). Nolte (1977) fed crowded Locusta migratoria hoppers a trisulpha antibiotic (sulphamethazine,
sulphathiazole and sulphapyridine) that presumably reduced the bacterial flora of the gut. These locusts had a significantly
lower chiasma frequency than crowded controls, indicating that microorganisms may synthesize locustol. Charnley, Hunt &
Dillon (1985) were able to rear Schistocerca gregaria axenically from sterilized eggs about as successfully as a stock
culture. They found that axenic locusts took twice as long to complete the last instar as groups of controls (in the stock
culture room) or groups of locusts that began axenically but were exposed to unfiltered air throughout life. This finding is not
supported by other workers (Nolte, 1976; Gillett & Phillips, 1977) who found that the gregarization pheromone from crowded
locusts caused a synchronization of molting time (relatively shorter last instar). Charnley et al. (1985) also reported that
axenic females were more typical of the solitary form than controls or the unfiltered air controls, supporting the idea that
bacteria may synthesize locustol (or other active component). However, a serious criticism of this work is their admission that
the crowded control group was reared in the same room as the stock culture and thus "may have been exposed to a greater
concentration of locustol" than the crowded axenic locusts "which were produced in relative isolation" in another room.
Further work is necessary to elucidate the role of the purported bacteria in synthesis of locustol and other possible
pheromone components.
(4) Reception
Two theories concern the site of sensory or physiological reaction to the gregarization pheromone or to locustol, (1) the
inhalation of locustol and induction of higher cAMP levels, and (2) antennal reception of locustol and brain mediation. Nolte
(1974, 1976) postulated that locustol is inhaled through the spiracles and ultimately may reach high enough concentrations in
the haemolymph to evoke behavioural and physiological reactions. The first theory developed when noradrenaline
(norepinephrine) was injected into fourth and fifth instar larvae of Locusta migratoria and raised the chiasma frequency
(Nolte, 1968). Other chemicals related to melanin, e.g. dopa, dopamine and protocatechuic acid, also raised the chiasma
frequency in albino but not in normal solitaries. As discussed earlier, chiasma frequencies may be directly affected by
melanin and colour changes and thus only correlated with the gregarization pheromone.
Solitary nymphs were injected with norepinephrine or locustol once at the beginning of the fourth instar and once at the
beginning of the fifth (Nolte, 1977). The mean chiasma frequency was raised from 10.5 (100%) to 12.4 (118%) by locustol
injection and similarly by norepinephrine. F/C ratios were also significantly affected. The combination of locustol and
norepinephrine had no effect compared to the control and was interpreted as indicating that the compounds were competitive
(and mimics) which he said was not surprising as their chemical structures are similar (norepinephrine is a catecholamine
while locustol is a substituted catechol). However, why should mimics or competitors, which alone are active, not be active
together?
Higher levels of cAMP were found in testes of adults that were crowded (locustol presumed present) than in solitary adults
(Nolte, 1977). This result, however, could mean either that locustol 'caused' cAMP increases (as Nolte supposes) or that
cAMP is the 'result' of phase changes. Nolte then injected hoppers as above with cAMP and found that chiasma frequencies
increased as well as a change in F/C ratio, but little effect was found on duration of the fifth instar. This result was interpreted
as evidence that locustol induced cAMP which then caused the phase transformation to gregaria. However, cAMP could still
cause transformation when cAMP levels are artificially increased by injection, but under natural conditions higher cAMP levels
may result from phase transformation. It would be interesting to determine the cAMP levels in locusts injected with locustol,
exposed to locustol vapours, and untreated.
The involvement of cAMP and locustol in gregarization may still be more complicated when one considers the role of
octopamine and stress during crowding of locusts. Stress from mechanical, heat or chemical injury causes the levels of the
neurohormone, octopamine, to increase in locusts (Davenport & Evans, 1984). One probable function of octopamine is to
boost the rate of glycolysis to prepare for strenuous demands of flight or defense. Injection of octopamine into locusts also
induces higher concentrations of cAMP in the haemolymph (Worm, 1980; Evans, 1985). Crowding may cause social conflicts
and stress which would raise the octopamine and cAMP levels to somehow promote gregarization. Further work is necessary
to elucidate the possible interactions of pheromone, octopamine, and cAMP in phase transformation.
The second theory is that the gregarization pheromone (locustol) is perceived by antennal receptors (Mordue, 1977; Gillett,
1983). Removal of antennae from crowded Schistocerca gregaria early in the third or fourth instar resulted in fifth
instar nymphs with green colour (mesobiliverdin) typical of solitary forms. The first quantitative study of colour was done by
extracting haemolymph and measuring the absorption at 650 nm (Mordue, 1977). When hind tarsi were removed or other
physical damage done as a control, no such effect in coloration occurred. Corpora allata from mature adults of Locusta
migratoria were implanted into fourth instar S. gregaria and caused haemolymph mesobiliverdin levels to increase
after 6 days. Mordue proposed that the antennae perceive the gregarization pheromone and inhibit the corpora allata from
releasing a hormone (juvenile hormone?) that induces the darker coloration and gregarious phase changes.
The injection of haemolymph from gregarious Schistocerca gregaria hoppers into solitarious ones caused them to
develop gregarious coloration, while injection of Ringer's solution or solitarious haemolymph had no effect (Nickerson, 1956).
The basic nature of this effect has not yet been explained. Studies of juvenile hormone titres and the role of phase
polymorphism in locusts have been recently reviewed by Dale and Tobe (1990). They conclude that "no very startling
evidence has yet been yielded by the comparative study of the action of endocrine agents in locusts of different
phases".
Gillett (1983) used second instar nymphs of S. gregaria and removed their antennae while they were reared crowded
(pheromone) or isolated, with or without nymphal faeces (pheromone). By the third instar, isolated nymphs without antennae
reared with nymphal faeces were less gregarious in behaviour and were differently coloured than controls with antennae,
which were more gregarious in the presence of pheromone from faeces. When isolated without faeces the removal of
antennae had no effect since no pheromone was present. Crowded nymphs without antennae did however show more
grouping, possibly due to social learning, but they were less likely to touch and had different coloration than crowded intact
nymphs. These results generally support Mordue (1977) that the antennae perceive the pheromone. However, Nolte's
theories arose while working with Locusta migratoria while Gillett and Mordue experimented with S.
gregaria.
A striking finding is that solitarious fifth instar and adult Locusta migratoria have more olfactory sensilla on the
antennae than do comparable gregarious phase insects (Greenwood & Chapman, 1984). In contrast, the numbers of trichoid
and coeloconic sensilla were not significantly different between the two phases. Greenwood and Chapman (1984) speculate
that the differences in olfactory sensilla may have evolved because individuals in groups (gregarious phase) require less
sensitivity to environmental stimuli due to the social reinforcing behaviour of others. One can also speculate that solitarious
individuals require more receptors in order to respond to the gregarization pheromone.
(5) Ecology
What is the advantage or purpose, from an individual's point of view, for the gregarization effect from the group? Why is the
morphological transformation adaptive? Obviously some physiological changes are advantageous in preparation for migration.
The ability to fly longer and more powerfully has been documented in gregarious forms (Michel, 1980; Nolte, 1977). In
pheromone communication it is proposed that both parties, sender and receiver, benefit (Burghardt, 1970). The advantage for
individuals producing pheromone is that all available locusts are 'recruited' to the migratory condition. The receivers benefit
as they should need to join the migration swarm. Both sender and receiver benefit if the fifth instar length is reduced and
molting is synchronized so that all individuals are able to leave in the swarm.
The reason why chiasma frequencies become higher in gregarious phase individuals has been attributed by Nolte (1973) to
be a beneficial increase in genetic recombination. Thus, "the offspring of migrations to new territories" will provide "genotypes
which might be found to be suitable for new adaptive requirements." In other words a solitary individual is by definition of its
existence rather well adapted to the local environment, where it has reproduced for several generations. But when preparing
to migrate it is advantageous to prepare sperm (and eggs if a female) that are even more genetically variable than usual
since it is not possible to predict which of the many possible habitats the migrant will settle in. With a larger ability to produce
variable gametes it is more likely, in theory, to produce a few progeny best adapted to the unique requirements of the newly
colonized land. One would predict that female oocytes also would show chiasma increases on exposure to the gregarization
pheromone. If this genetic recombination hypothesis is correct, then it could be expected that nearly all long-range dispersing
insects exhibit increased chiasma frequencies in the generation that founds colonies in new breeding habitats.
Mordue (1977) describes the colour of gregarious Schistocerca gregaria as having an "orange, pink and yellow
background superimposed with a well-developed black pattern" while solitary forms at endemic population levels are 'cryptic'
with green cuticle and haemolymph and no black pattern (Ellis, 1951). Kennedy (1962) and Lea (1962) have suggested the
colouration of the gregarious form is aposematic (bright warning colours indicating a poisonous insect). Gillett (1973) criticises
this hypothesis since the nymphs are palatable to birds, insects, reptiles, spiders, amphibians and many mammals, including
humans (Ashall & Ellis, 1961; Roffey & Popov, 1968; Stower & Greathead, 1969; Shurney & Zottola, 1976). Experiments with
albino nymphs and normal S. gregaria showed no preferential grouping with either colour form, indicating that visual
stimuli regarding colour patterns were not important in forming aggregations (Gillett, 1973). This led her to conclude that the
colour pattern is not aposematic or Batesian mimicry (not poisonous but colour/form mimicking a poisonous species) since
the ratio of mimics to models in locust swarms must greatly exceed 4 to 1. Brower (1960) found mimics derive some
protection from birds if the mimics outnumber their models by a ratio of up to 4:1. Gillett further argued that for locusts to
derive a benefit any models would need to occur over a wide range of habitats of migrating locusts and no such model has
been proposed.
However, one can suppose that the gregarious coloration is advantageous to locusts as a form of non-specific Batesian
mimicry. All can agree that there are many brightly coloured insects that are poisonous such as wasps and bees in a broad
diversity of habitats. A locust swarm is ephemeral in space and time, and habituation by local predators might not occur for
some time after a swarm arrived. The very nature of a swarm of 'aggressive' and 'aposematic' insects buzzing loudly could
frighten many predators away from the area.
Individual locusts when flying in proximity to others do have a common orientation that can be at almost any angle to the
wind, but the swarm usually migrates downwind (Waloff, 1972; Riley, 1975; Riley & Reynolds, 1983; Baker et al., 1984;
Rainey, 1989; Farrow, 1990). Individual locusts (probably Locusta migratoria) in a swarm at night were seen by radar
to be flying with a similar body orientation (Riley, 1975). Observations of locust swarms have stated that the orientation of
individuals at the swarm periphery were deflected inward toward the center of mass (Rainey & Sayer 1953; Haskell, 1957).
The turning back into the swarm was reported by Haskell (1957) to range from 2-23 m outside the swarm, and from 20-80 m
by Waloff (1972). The swarm cohesion is believed to be maintained by visual cues and at closer range also by acoustic
stimuli at 4-8 m range (Haskell, 1957). Locust swarms may migrate slowly and even appear stationary for many hours
(Rainey, 1989) and individuals may spend up to 90% of their time on the ground (Farrow, 1990). Waloff (1972) has
questioned whether a swarm cohesion pheromone might exist.
What is the advantage of swarming as opposed to migrating singly? As mentioned above, a swarm of seemingly aposematic
insects might be more threatening than just one such insect. If it is assumed that locusts derive a benefit from migration in a
certain direction as do birds then one theory is that a single animal is more likely to be inaccurate in orienting in a preferred
direction than is a flock or swarm that uses the mean direction (Hamilton, 1967; Wallraff, 1978). However, although locusts
probably migrate using a mean direction of many individuals' responses held together by acoustic responses (Michel, 1971;
Yinon, Shulov & Tsvilich, 1971) there is no evidence for orientation to compass or sun direction (Baker, Gewecke & Cooter,
1984).
It might also be that individuals derive benefits by flying in a concentrated mass so that territorial predators are locally
satiated and the probability of an individual's capture is thus reduced. Another theory is that the swarming individuals
'confuse' a predator and make it less likely that any particular individual is captured (Bertram, 1978). Finally, if a long-range
sex pheromone is lacking (as appears the case) then locusts that could remain in groups during and after migration would
have advantages in finding mates. Field observations and experiments are necessary to investigate these hypotheses.
III. ADULT MATURATION PHEROMONE
A maturation pheromone was demonstrated for Schistocerca gregaria by Norris (1954). Immature males change from
pinkish-brown to uniformly yellow as they become sexually mature. Mature yellow males when placed with immature males
and females accelerate the latter's maturation process. Loher (1958) then reported that parts of mature males or oil extracts
held in front of antennae of immature adults of either sex released a 'vibration reaction', the "antennae begin to move in a
non-directional manner, and this is followed by strong agitation of the two pairs of palpi," then "the hind femora begin to
vibrate rapidly . . ." The vibration reaction was reported to contain no acoustical component. Little data were reported until the
full report by Loher (1960), which found a correlation between the olfactory vibration stimulus and the maturation
pheromone.
(1) Vibration reaction
The vibration reaction was observed often in immature locusts of either sex when a yellow mature male approached (Loher,
1960). Covering the eyes and ocelli with black varnish did not prevent the vibration reaction when a mature male was placed
within 1 cm of the blindfolded locust. Visual stimuli alone from mature males placed in transparent airtight glass tubes were
not effective in releasing the vibration reaction. Air passed over mature males and then through a tube to the antennae of
immatures caused the vibration reaction while pure air puffs were ineffective. Mineral oil extracts of mature males placed on
cotton-wool and offered to immature locusts elicited the vibration rather consistently in over 1000 tests while control oil gave
no reaction. Only the oil extracts of mature males were effective, neither young nor mature females nor young males elicited
the vibration reaction. However, Amerasinghe (1978a) was unable to reliably invoke the vibration response with mature male
extract in his strain of Schistocerca gregaria. Instead he describes a 'freezing' or avoidance reaction, if one occurs at
all.
(2) Reception
The antennae appear to be the receptor for the vibration stimulus since removal of antennae from 50 males and 20 females
prevented the vibration response to active extracts in all cases (Loher, 1960). On the other hand, removal of palpi from 20
males still allowed the response in every case when offered a yellow male. Even dilution of a one mature male extract by
5000 gave a vibration reaction in 18 of 31 males while oil alone gave no such reaction. Loher (1960) found that diethyl ether
was efficient in extracting the stimulus from locust males but that after the solvent evaporated the stimulus remained on
cotton-wool for a "short time" indicating a significant degree of volatility. Mineral oil was found to be a convenient way of
retarding the volatility of the active substance for longer term experiments on sexual maturation. It should be emphasized that
the chemical stimulus for the vibration reaction may not necessarily be the same as that promoting yellowing or sexual
maturation. Amerasinghe (1978a) found ether extracts of mature males induced sexual maturation (willingness to mate) but
these were inconsistent compared to living mature males in causing the yellowing process. A pheromone isolation strategy
based on the vibration reaction would provide a convenient and consistent bioassay but this bioassay may not be relevant to
sexual maturation. A substance could be isolated that evokes vibration, and nothing more.
Short exposure of sexually immature males to within 1 cm (but not touching) of mature males each day for 20-30 days
caused most test males to copulate with females while control males did not copulate (Loher, 1960). Also the yellow
coloration of the treated males was more pronounced than in the controls. A similar test with oil extract of mature males on
cotton-wool caused test males to accelerate the yellowing process compared to the control group. Males of Locusta
migratoria did not appear to cause sexual maturation in Schistocerca gregaria. It was not possible to determine if
the maturation pheromone is received by the antennae since their amputation, or other body damage, caused an acceleration
of maturation.
(3) Source and physiological regulation
Loher (1960) also found differences in the Schistocerca gregaria epidermis between mature males and young males
or females that were correlated with the presence of the maturation pheromone. Mature males have about a three times
thicker epidermis than immature males. The epidermal cells of mature males also show many vacuolated cells that are
presumed to hold the maturation pheromone. Gregarious form females, either young or mature, had an epidermis similar to
young males. Mature males of S. gregaria in the solitary phase and mature Locusta migratoria males have a
thicker epidermis but the cells are not vacuolated. These findings have been confirmed for S. gregaria (Thomas, 1970;
Strong, 1970, 1971).
The number and distribution of glandular cells (vacuolated cells) is greater and more widespread in crowded males than in
isolated males (Thomas, 1970). Thus one can postulate that the maturation pheromone is stimulated by the gregarization
pheromone. Strong (1970) also reported vacuolated cells in gregarious mature males of Schistocerca gregaria and
states that, contrary to the earlier belief that the vacuolated cells arise from the original epidermis, the vacuolated cells grow
as additional cells. Coiled ducts have been found that appear to connect the vacuolated cells with the outer surface,
presumably for release of the maturation pheromone (Thomas, 1970; Strong, 1971; Kendall, 1972). Mature males have an
aromatic smell (Norris, 1954) but attempts to identify the responsible chemicals, or the pheromone, have been inconclusive.
Blight (1969) collected air from locusts and identified an unusual amine, 1-pyrroline, associated only with mature males.
Further tests were proposed by Blight (1969) in order to determine the role of the sex-specific volatile but have apparently
never been published.
In still more experiments, Loher (1960) studied the role of the corpora allata in sexual maturation. He first removed gonads
and accessory glands but these had no effect upon maturation. The histological sectioning of the corpora allata, however,
revealed that in mature males these glands were active and produced "abundant secretion". Removal of corpora allata from
immature males that were reared crowded inhibited accessory gland development, as well as the normal change to yellow
colour and normal sexual behaviour. These allatectomized males also appeared to lack the maturation pheromone since they
could not evoke the vibration response in other locusts. Most importantly, the epidermis of allatectomized males was
indistinguishable from young immature males. These changes could be substantially reproduced even with already mature
males, which subsequently lost coloration and vacuolated cells and were unable to release the vibration response. This
indicates that a continuous supply of hormone is required to preserve sexual maturation.
Loher (1960) implanted corpora allata into previously allatectomized males that had lost coloration and glandular epidermal
cells as well as sexual capacity. After implantation, the males became yellow, copulated freely, and produced epidermal
secretion from the vacuolated cells. However, the males with implanted corpora allata needed the presence of other males to
provide a source of maturation pheromone. It is not clear how important the maturation pheromone is compared to the
gregarization pheromone is this case. The effects of removal and implantation of corpora allata were confirmed by several
workers (Pener, 1965, 1967a, b; Odhiambo, 1966). However, Cassier & Delorme-Joule (1976) are in conflict with the above
studies on one point. They report that corpora allata are more active in hormone secretion in solitary males than in crowded
males. They propose that ecdysone promotes gregarious development while the presence of ecdysone and juvenile hormone
induces solitarious type development.
Amerasinghe (1978b) assumes the corpora allata secrete one or more juvenile hormones (J.H.) as "this is well established."
Injection of J.H. I (3,11-dimethyl-10-epoxy-7-ethyl-trans, trans-2,6-tridecadienoic acid, methyl ester) and J. H. III (10-
epoxy-3,7,11-trimethyl-2,6-trans-dodecadienoic acid, methyl ester) into crowded allatectomized males stimulated
yellowing, with J.H. I being more effective. As found also for corpora allata, the juvenile hormones are not effective in
restoring sexual maturation when insects are kept isolated (Loher, 1960; Amerasinghe, 1978b). The male-specific maturation
pheromone was produced by J.H. injection in allatectomized males. However, the effect of females or immature males on
J.H. treated allatectomized males was not determined (Amerasinghe, 1978b), which might have proven whether the effects
were due to the maturation pheromone or to the gregarization pheromone. Amerasinghe (1978b) refers to his Ph.D. thesis in
which extracts of the maturation pheromone were not effective on accelerating maturation in isolated locusts, indicating the
gregarization pheromone is necessary. Only J.H. III has been found in Schistocerca gregaria and the doses of
hormones were far in excess of natural estimates, although such doses may be necessary due to excretion and the
requirement for physiological action over a longer period of time (Amerasinghe, 1978b).
A maturation pheromone has also been found in male Locusta migratoria (Norris, 1964). The time from molting to first
copulation was noted for each male in groups of 10 pairs that had two mature males or not. The immature males in groups
without maturation pheromone from mature males took significantly longer to attain sexual maturity (about 19-25 days
compared with 13-14 days). Little else has been done to elucidate this phenomenon.
Norris (1962, 1964) also proposes that in both species a pheromone inhibiting male maturation is released by young nymphs
while a maturation pheromone is released by mature adults - thus maturation of the group is synchronized. There is some
evidence that adult males crowded with young nymphs took longer to mature sexually than those reared isolated (Norris,
1962). The effect may not be due to an anti-maturation pheromone but simply due to effects of crowding on feeding
behaviour or even due to the gregarization pheromone. The use of extracts of nymphs presented to isolated immature adult
males might settle the question of the existence of the anti-maturation pheromone.
(4) Ecology
Norris (1962, 1964) presents a theory that the maturation-retarding effects of crowding with nymphs are counteracted by the
accelerating effect of mature males such that maturation is thus synchronized. Since maturation in solitary males cannot be
accelerated by the maturation pheromone it seems that the gregarization pheromone is also involved, a fact ignored
previously. The benefits for mature individuals to release a maturation pheromone, if they are gregarious (hence the
gregarization pheromone must be involved), that promotes group maturation are the same as discussed above for the
gregarization pheromone. Similarly, the receivers should need to mature rapidly if many gregarious mature adults are present
and preparing to migrate. The anti-maturation pheromone would benefit receiving adults who would need to wait until the
many nymphs had completed development. It seems important to investigate the maturation of females (Norris, 1954;
Highnam & Lusis, 1962) since they should be affected similarly as immature males in regard to maturation pheromone
released by males and anti-maturation pheromones released by nymphs.
Carlisle, Ellis & Betts (1965) provide another explanation for the synchronization of adult maturation in Schistocerca
gregaria. They report extensive data that locust maturation coincides with the bud burst of certain desert shrubs, a week
or more before the heavy rains begin and several weeks before the appearance of the annual vegetation. The shrubs
included many species of Boswellia and Commiphora which have resinous buds that are the source of the
biblical frankincense and myrrh. These essential oils contain monoterpenes, among other compounds. Buds of the shrubs
when placed in a cage with locusts caused them to mature more rapidly than controls as evidenced by colouration changes.
The monoterpenes, ā-pinene and á-pinene, found in myrrh when applied to locusts were effective in promoting and
synchronizing maturation (Carlisle et al., 1965). These authors did not consider the possibility of a maturation pheromone,
although their work does not rule one out. It is possible that volatile monoterpenes serve as a signal for the induction of
maturation pheromone which then stimulates maturation.
It should also be pointed out that maturation rate is enhanced when locusts are injured or stressed by forced activity (Norris,
1954; Highnam & Haskell, 1964). These confounding effects may have influenced results of earlier studies on maturation
pheromone. As mentioned earlier, octopamine and cAMP levels may be increased due to stress and forced activity
(Davenport & Evans, 1984; Evans 1985), and these compounds may have mimicked or interacted with the effects of the
maturation pheromone.
IV. OVIPOSITION-STIMULATING PHEROMONE
Oviposition by female Schistocerca gregaria is stimulated by copulation (Norris, 1954). Pickford, Ewen & Gillott (1969)
working with the migratory grasshopper, Melanoplus sanguinipes, showed that an egg-laying stimulant was produced
by the male accessory reproductive glands. In S. gregaria, Leahy (1973) implanted male accessory glands in virgin
females and found their oviposition rate could be increased. Lange & Loughton (1985) showed that injection of mature male
accessory gland extracts of Locusta migratoria stimulated an increase in oviposition rate of virgin females comparable
to that of mated females.
The accessory gland is composed of 16 pairs of tubules, of which tubule #1 is called the opalescent gland (Odhiambo,
1969). Only the opalescent gland or the spermatophore (sperm packet) were active in stimulating oviposition, therefore a
substance is transferred to the female through the spermatophore during mating (Lange & Loughton, 1985). The pheromone
appears to be proteinaceous with a molecular weight of 13000 as determined by sephadex gel filtration. The molecule
contains high amounts of lysine and may interact with the corpus cardiacum since injection of only 4% of this body into virgin
females stimulated oviposition (Lange & Loughton, 1985). Earlier, Okelo (1971) with Schistocerca gregaria showed
that oviposition is stimulated by a blood-borne factor, since egg-laying was induced in females injected with haemolymph
from ovipositing females.
V. OVIPOSITION-AGGREGATING PHEROMONE
Females of Schistocerca gregaria tend to lay their egg-pods in areas where other females are ovipositing even
though other areas had environmental factors that appeared suitable (Popov, 1958; Stower et al., 1958). Norris (1963)
explored this phenomenon in the laboratory. She found that females oviposited more in sandy areas with living decoys
(locusts of either sex tethered with fine wire) than in areas without decoys. The ovipositional preference for areas among
decoys occurred in both light and dark. Paper decoys were ineffective unless the paper was taken from cultures of locusts.
Mature males of Locusta migratoria were effective as decoys only when alive and could not compete with locusts of
S. gregaria. When used as decoys, freshly killed S. gregaria were as effective as living locusts in the dark.
Ether extracts of mature adults usually were effective in the oviposition bioassay, but no further progress in isolation has
been made. These results indicate a species-specific pheromone present on the body of both males and females of S.
gregaria. The effect from living decoys in the dark was even able to overcome the female's usual preference for moister
sand in which to oviposit. Many insects have been shown to possess oviposition-stimulating or deterring pheromones but
very few chemicals have been identified until recently (Hurter et al. 1987; Ali & Morgan, 1990; Prokopy, 1981)
(1) Reception and source
Removal of the antennae of females only diminished slightly the responses to dead decoys in the dark. The lower
locomotory activity of antennectomized females was believed to lessen their chances of coming within effective range of the
decoys. However, removal of the antennae greatly diminished response to decoys screened within cages (Norris, 1963,
1970). Norris (1970) supposes that chemotactile receptors on other parts of the body in addition to the antennae and possibly
the palpi are able to function sufficiently well to promote oviposition. Chemoreceptors on the dorsal and ventral valves of the
ovipositor have been described for Schistocerca gregaria by Thomas (1965). Contact chemoreceptors of locusts also
occur on the tarsi, mouthparts, and the antennae (Chapman 1982; Blaney & Simmonds, 1990).
In other experiments Norris (1963) demonstrated that isolated females were not able to respond to the oviposition-
aggregating pheromone released by live decoys in the dark. Since the other tests used locusts reared in groups this implies
the gregarization pheromone must predispose females to respond to the oviposition-aggregating pheromone. In a subsequent
report, Norris (1970) tested dead locust decoys that had been reared isolated and found they had less oviposition-
aggregating pheromone than dead decoys reared crowded. However, the isolated decoys when compared with Locusta
migratoria decoys were more stimulatory to ovipositing females. Heads, thoraxes and abdomens were all effective in
eliciting oviposition of females. Observations of females searching for oviposition sites have been described by Norris (1963),
"the females seemed to enter the decoy group accidentally as a result of random wandering" and any attraction was exerted
only at a distance of a few inches at most. There is no reference that females touched the decoys with their antennae. L.
migratoria females in the dark also appear to respond to decoys of their own species preferentially over S.
gregaria, however, the response to oviposition-aggregation pheromone seems weaker than those normally found for
S. gregaria (Norris, 1970).
An interesting comment by Norris (1970) is that "response to extracts had been deteriorating since the first experiments were
carried out and the possibility has to be considered that there had over the years been unconscious selection against
responsiveness in the locust stocks at the Anti-Locust Research Centre." In fact, the artificial breeding of locusts in groups
much smaller than natural populations should lead to genetic drift as well as loss of heterogeneity (once a gene is lost due to
chance it cannot be reintroduced as in natural populations by immigration). It is also possible that artificial selection for traits
adapted to the conditions in the laboratory resulted in unfortunate changes in genetic frequencies of natural traits of interest
by means of genetic linkages. This is a common, and largely unaddressed, problem with insect cultures that may be more
significant than realized. In general, laboratory stocks should be replaced (not added to) with field collections after several
generations. The number of recommended generations is difficult to estimate, however, since it depends on the strengths of
the artificial selection pressures (which are unknown) and on the size of the laboratory population.
In contrast to Schistocerca gregaria, Locusta migratoria females of both phases are attracted over a distance
of 0.5 m to sand into which gregarious females had laid egg pods (Lauga & Hatte, 1977). The females were reported to
ingest sand. An accumulation of an oviposition-aggregation pheromone occurred as the attractiveness of the sand increased
with more frequent use for oviposition. The attractiveness lasted for up to 6 months. Lauga & Hatte (1978) found that when
solitary females were given attractive sand, the number of egg pods increased like in gregarious females, as well as the
weight per egg. The active sand caused females to lay eggs that hatched in a more gregarious form than if laid in clean
sand. The antennae and palpi may detect the pheromone. It is not known if the aggregating pheromone consists of the same
components as the primer pheromone that alters the physiology of ovulation leading to egg production of the gregarious
type.
(2) Ecology
Norris (1963) wondered if the oviposition-aggregating pheromone "may not always be advantageous" since females could be
fooled by decoys into laying eggs in sandy areas of suboptimal moisture where eggs would not hatch. However, tethered
decoys are not natural and it must be assumed that the first females will choose appropriate sandy areas. Also, it may not be
possible for females to predict the future moisture conditions at the oviposition site that would depend on future precipitation.
The phase of Schistocerca gregaria hatchlings is not determined by a pheromone but rather by the moisture content
of the sand surrounding egg pods. Sand of 24% moisture produced mostly black hatchlings of the gregarious form while sand
moisture of only 2% produced solitary form hatchlings (Hunter-Jones, 1962). Norris (1963) expressed the belief that the habit
of gregarious oviposition is adaptive because the young hoppers upon hatching are aggregated so that gregariousness is
perpetuated. As discussed earlier, remaining in groups may be beneficial in terms of preparation for migration, avoidance of
predators, and finding mates.
VI. OTHER POSSIBLE PHEROMONES AND SEMIOCHEMICALS
A swarm of gregarious phase locusts may contain millions of individuals per km2 (Singh & Singh, 1977). Solitary-
form populations of Schistocerca gregaria in India have been estimated at various places to range from 25 to 20,000
per km2 (Harjai, 1974). At high densities it should be easy for males to locate females by random wandering, but at
densities of a few tens or hundreds per km2 it seems that the population would become extinct (maybe it does)
unless a long-rang sex pheromone is used. However, Haskell, Paskin & Moorhouse (1962) found no evidence of an upwind
attraction to locust volatiles under the conditions they employed. According to Whitman (1990) there are only two
grasshopper species that have been rigorously shown to possess sexual pheromones. Hieroglyphus nigrorepletus
males use their antennae in attraction to females over several centimeters distance (Siddiqi and Khan, 1981). In
Taeniopoda eques a contact sex pheromone from females, detected by the male's antennae, causes males to attempt
copulation (Whitman, 1982). No chemical structures have yet been identified.
Many other insects use a long-range pheromone (usually attraction of a meter or more) to find mates or mates at food
resources (Baker, 1989; Byers, 1989; Ali & Morgan, 1990). It would be interesting to apply a recent mate-finding rate model
(Byers, 1991) to locusts to see if they require a long-range pheromone during the solitary phase. The model uses the
parameters of insect density (number per area), apparent detection radius (i.e. attraction to pheromone), time of search, and
walking/flying speed to determine mate-finding success.
In groups of locusts a long-range sex pheromone seems unnecessary, although a sex recognition pheromone is evident.
Females of Schistocerca gregaria are passive, displaying no calling behaviour, and appear to be forcibly copulated
with by males (Norris, 1964; Amerasinghe 1978b; Strong & Amerasinghe, 1977). Males, however, seem to recognize females
due to some contact sex-pheromone. Norris (1962) reported that when mature males are crowded together without females
they sometimes mount backs of males and attempt copulation. Interestingly, isolated and thus more solitary form males were
the ones most frequently selected by gregarious form males. Apparently, the crowded males, which naturally would not be
exposed to solitary males, were less able to differentiate solitary males from females, while gregarious males were
recognized as males. The absence of male maturation pheromone on bodies of females and solitary form males could be the
cue, or a sex pheromone on females, as they were preferred most. In addition to the maturation pheromone, sexual
dimorphism has been observed in cuticular proteins of Locusta migratoria (Cassier & Papillon, 1983; Cassier et al.,
1980) which indicate that chemical differences are available for sexual discrimination during copulatory attempts. Peschke
(1987) showed that cuticular hydrocarbons served as cues for sexual recognition in a rove beetle (Staphylinidae). Few
studies have identified the contact pheromones that indicate sex and species other than those with moths (Baker, 1989;
Byers, 1989; Ali & Morgan 1990; Peschke, 1987).
Haskell et al. (1962) showed that Schistocerca gregaria hoppers wandered downwind in a wind tunnel in clean air but
immediately orientated and then walked upwind in response to the introduction of grass odors. Kennedy & Moorhouse (1969)
showed that the attractive response to odors was anemotaxis since one antenna, or crossed-over antennae, still allowed
upwind response. Locusta migratoria has been shown to possess single olfactory receptor cells on the antennae
which respond to enantiomers of 4-methyl hexanoic acid, more so to the (-)-isomer, at concentrations of only 2
ng/cm3 (Kafka et al., 1973). It is not known if these enantiomers are biologically relevant but it does indicate that
receptors exist for enantiomeric discrimination of compounds. Locustol, guaiacol, and phenol, purported locust pheromone
components, contain no asymmetric carbon atoms and thus do not possess enantiomeric forms (Fig. 2).
Norris (1962) reports that attraction to "grass odor" can occur on a micro-environmental level, the "locusts sat or crawled in
the vicinity of the food plant for long periods without feeding until one would fortuitously encounter a leaf and begin to feed.
Almost at once several others would approach the plant and feed . . .". Upwind attraction of Schistocerca gregaria to
grass, cabbage, or privet odors in a laboratory arena has also been reported (Haskell et al., 1962). It may be important to test
olfactory responses at appropriate times of the day since Ellis & Ashall (1957) showed there was a diurnal rhythm of feeding.
No plants have been reported to repel locusts from a distance of several centimeters or more. However, vapours of carbon
tetrachloride or valeric acid induce anemotaxis downwind (Haskell et al., 1962; Kennedy & Moorhouse, 1969).
The antennae of Locusta migratoria have olfactory receptors of two types, types A and B (Ameismeier, 1987). Type A
has from 20 to 30 neurones (Ameismeier, 1987), suggesting that this receptor probably functions in cognition of plant odours.
Type B has only three neurones, more typical of receptors responding to pheromone components. Greenwood and Chapman
(1984) did not observe significant differences in the distribution and abundance of olfactory receptors on the antennae
between males and females. As mentioned earlier, they did find significantly more of type A and type B receptors on the
solitarious adult than the gregarious adult. The solitarious adult, being at low densities, would require more receptors and
sensitivity for locating mates (type B as proposed here) and possibly also food plants (Type A). Locusts have chemoreceptors
on the tarsi, mouthparts, and antennae which serve in the detection of suitable food (Thomas, 1966; Chapman, 1982;
Greenwood & Chapman, 1984; Ameismeier, 1987; Blaney & Simmonds, 1990).
Many plant species contain feeding deterrents, including terpenes (limonene, geraniol, citral and azadirachtin), non-protein
amino acids, and alkaloids (Butterworth & Morgan, 1971; Gill & Lewis, 1971; Navon & Bernays, 1978; Ohabuike, 1979; Evans
& Bell, 1979; Mwangi, 1982; Singh, 1983; Monache, Bettolo & Bernays, 1984). Adams & Bernays (1978) found that 14
feeding deterrents when presented individually at lower dosages were not deterrent but when combined they were deterrent.
It is interesting to note that the polyphagous Schistocerca americana can acquire taste aversions for palatable plants
by learning to associate an illness-inducing injection of nicotine hydrogen tartrate (NHT) (Bernays & Lee, 1988; Lee &
Bernays, 1990). However, the food aversion learning was non-existent with preferred foods such as broccoli compared with
immediate learning with a less acceptable food (spinach). A potential problem for control applications with deterrents is that
central nervous habituation to at least some feeding deterrents can occur in S. gregaria (Szentesi & Bernays, 1984) as
has been shown in other insects (Chapman, 1974).
Feeding stimulants have been isolated from a host plant of Schistocerca gregaria but not identified except for sucrose
(Rao & Mehrotra, 1977; Rao, 1982). Of a range of nutrient chemicals only hexose and disaccharide sugars were highly
stimulatory while L-proline and L-serine elicited some feeding response (Cook, 1977). Chemoreception studies of locust
mouthparts have shown that most sensilla respond to a wide variety of compounds (Haskell & Schoonhoven, 1968; Blaney,
1975, 1980; Winstanley & Blaney, 1978). Recently it has also been shown that S. americana have tarsal receptors
sensitive to various chemicals (White & Chapman, 1990). NaCl appeared to be detected generally by tarsal receptors,
sucrose weakly, while specific neurons received NHT but not NaCl.
VII. CONTROL
Integrated insect pest management involves the use of more than one and usually several methods designed to reduce
damage to plants. One primary strategy that has been employed extensively is the use of general factors that affect insect
biology such as neurotransmitters, hormones, and unspecific predators and parasites, to formulate a population control
method. The other main strategy has been to investigate the pest species system of interest in order to find uniquely specific
components that can be manipulated or weakened resulting in mortality of the pest population. The problem with the first
strategy that uses pesticides, hormones, and general insect enemies is that it has the potential to affect many insects and
other organisms in the ecosystem with unknown consequences.
While all knowledge is useful to varying degrees when designing integrated pest management programs, it should be
considered best to concentrate on species-specific or group-specific physiological, behavioural, and ecological aspects that
can be interfered with in a manipulative way. For example, releasing a natural or molecular engineered pathogen into the
environment is not manipulative if the pathogen becomes established. The pathogen is potentially available for colonization of
other species without regulation by man. On the other hand, if the pathogen kills the pest and then dies out, this would be
considered manipulative since man has control over the use of the pathogen. Pesticides are then manipulative but of course
have the disadvantage of upsetting the ecosystem because of their non-specific affects on a wide variety of species. The
great advantage of the use of pheromones and other semiochemicals is that they are both manipulative and species-specific.
In addition they are generally non-toxic and at the concentrations envisioned for use in control programs would be expected
to have virtually no adverse affects on any other organisms in the ecosystem.
The use of feeding deterrents and stimulants would be manipulative but deterrents may be distasteful or toxic to humans as
well as to locusts. Grainge and Ahmed (1988) have compiled a list of all known plants (and references) which are noxious to
locusts as well as many other insects. Locusta migratoria has so far been found to be deterred by 56 plant species, at
least 60% containing alkaloids, while Schistocerca gregaria is deterred by 19 plants, also mostly containing alkaloids.
One major problem with deterrents is that crop plants must be treated uniformly with a generally non-volatile compound of
long persistence; this may be impractical. Feeding stimulants in baits containing biological agents or insecticides are also of
limited value unless a long-range attractant can be added. Otherwise, locusts would not find the limited number of baits; or a
high density of baits would be required making the strategy impractical.
Speculation on the use of locust pheromones in integrated control programs has focused on disrupting the gregarization
process during favorable climatic conditions and preventing the damaging swarms of migrating locusts. Thus the
gregarization pheromone must somehow be countered. The evidence for a solitarizing pheromone produced by adults (Gillett
& Phillips, 1977; Gillett, 1983) is weak and thus not promising. An anti-maturation pheromone produced by very young
nymphs as suggested by Norris (1962) also may not exist and is of dubious value. Even if these pheromones are proven and
eventually isolated they probably will not be able to overcome the natural gregarizing pheromone produced by dense
populations of locusts. However, more research should be done to delineate the existence of these pheromones and their
ability to inhibit the gregarization and maturation pheromones.
More promising, but little considered until now, is the use of gregarizing pheromone and maturation pheromone to cause low
density populations of solitary form individuals to undergo phase transformation and migrate prematurely. It can be argued
that migration by locust individuals is disadvantageous since predators may be more effective than when migration is in
swarms. Pheromones could also be used during unfavorable conditions for migration. Also it can be hypothesized that
pheromone application on locusts at low population levels would cause inopportune migration and dispersion of individuals so
widely that mate finding would be nearly impossible.
Similar reasoning makes a case for the oviposition-aggregating pheromone as being very promising for control. Here two
methods can be experimented with once components are isolated and identified. The first strategy would be to add either
insecticides or parasites to a sandy area treated with pheromone, thus killing either the adults with a fast acting insecticide or
the eggs/nymphs with a persistent one. Unfortunately, insecticides are often repellent to insects so this may be a drawback,
in addition to the other problems with toxic substances. Nematodes able to kill eggs (Khan, 1979) might be placed with the
pheromone. The second strategy would be to spread the oviposition-aggregating pheromone over a wide area to disperse the
egg laying so no groups were formed. The advantages of grouping, discussed previously, would thus be negated. The
oviposition-aggregating pheromone, once identified, could also be used in traps for monitoring populations. Isolation and
identification of the known locust pheromones, and possibly some new ones as suggested above, is the prerequisite to
further experiments using pheromones in direct control and monitoring population levels.
VIII. CONCLUSIONS
Most research on the various pheromones of locusts has been undertaken in the laboratory while confirmatory and
complementary studies in the field have been lacking. There is strong evidence for a gregarization pheromone that volatilizes
from faeces of both sexes in dense populations. The pheromone effects the physiological transformation from solitary forms
to gregarious forms which have gregarious behaviour, darker colour and different morphological dimensions. However,
reported effects of the gregarization pheromone on chiasma frequencies during meiosis have not been confirmed by
independent workers. There has not been a rigorous isolation and identification of the pheromone despite reports that
locustol and various analogs are biologically active. Reported evidence of a counteracting pheromone or solitarization
pheromone have not been substantiated. The advantage of a gregarization pheromone to the sender and receiver is that a
large group is formed for migration which presumably is protected from predation and may also orient more accurately than
migrating individuals would alone. An adult maturation pheromone affecting immatures of both sexes and produced by adult
males, and accumulating on the cuticular surface, has been established although the responsible chemical compounds are
not known. Plant compounds, including monoterpenes, from desert shrubs may induce development of the maturation
pheromone in natural populations of locust. The benefits to individuals in a dense population are that they reach maturity in
synchrony and are thus ready to migrate en masse. An oviposition-stimulating pheromone produced by male accessory
reproductive glands and transferred during copulation is known but the amino acid sequence of the protein has not been
determined. A volatile oviposition-aggregating pheromone produced by both sexes elicits females to oviposit in areas of
pheromone release. The responsible semiochemicals have not been identified. An oviposition-aggregating pheromone would
benefit individuals by keeping the immature hoppers together from the start so that if densities were high the gregarization
pheromone could exert its beneficial phase-transformation effects in preparation for migration. Other possible pheromones of
locusts that would be used in long-range sex attraction and in close-range sex recognition should be looked for. A promising
control strategy is the use of the gregarization and maturation pheromones to cause a low density population to migrate
prematurely or at an inopportune time. Using similar reasoning, the oviposition-aggregating pheromone could be used to
disperse the oviposition sites to the detriment of the grouping of gregarious phase populations. Alternatively, the pheromone
could be used to concentrate the locusts where one or more pathogens or insect enemies could cause high mortality.
IX. SUMMARY
Modern studies of chemical ecology and behaviour of the locusts Schistocerca gregaria and Locusta migratoria
in the laboratory need to be more closely coupled with field experiments and observations. The life history relating to
oviposition, transformation to gregarious phases, and adult maturation mediated by pheromones is reviewed. The principles
of pheromone isolation and identification are discussed. The long-term effects of the gregarization pheromone on the
physiology are presented with discussion of morphological changes, chiasma frequency increases, and synchronization of
molting induced by the pheromone. Isolation of the purported gregarization pheromone, locustol, from faeces is discussed in
regard to inconsistent effects. Other more immediate effects of the pheromone on the social (gregarious) behaviour and the
isolation of possible pheromone components different from but related to locustol are presented. It is stressed that more
rigorous isolation studies should be undertaken due to the conflicting reports and methodological problems. The possibility of
an anti-gregarization pheromone or solitarizing pheromone is discounted. The source and biosynthesis of locustol (or
gregarization pheromone) from degradation of lignin by symbiotic bacteria is discussed. Theories of reception of the
gregarization pheromone such as inhalation through the spiracles or sensory perception by the antennae are presented. Also
an internal mechanism involving cAMP and/or corpora allata may be induced by gregarization pheromone to effect the
physiological phase changes. The ecology in terms of the advantages of an individual in reception of the gregarization
pheromone from a group of gregarious and pre-migrating locusts is discussed. Also the possible benefits of gregarious
behaviour, phase polymorphism and migration are dealt with.
An adult (sexual) maturation pheromone has long-term effects on reducing the period of maturation and immediate effects on
the behavioural vibration response. The epidermal source of the pheromone and glandular cells responsible for the
production of the pheromone are discussed. The reception and internal mechanisms of response via the corpora allata are
mentioned. The ecology regarding the benefits to individuals for the synchronization and rapid maturation of adult maturation
in a gregarious group is considered.
An oviposition-stimulating pheromone produced by the male accessory reproductive glands appears to be a proteinaceous
substance of large molecular weight. On the other hand, an oviposition-aggregating pheromone volatilizes from epidermal
areas of either sex and causes higher oviposition rates in the area of release. The behavioural and ecological aspects of this
pheromone are discussed. Several other possible pheromones and semiochemicals are discussed such as a long-range sex
pheromone, sex-recognition pheromone, grass odors and feeding stimulants and deterrents. Several possible control
strategies using locust pheromones are considered. The general conclusion is that the chemical isolation of the various
pheromones is necessary before further progress can be achieved on the source and biosynthesis of pheromone, reception
of pheromone, behavioural effects of pheromone, and control measures.
X. ACKNOWLEDGEMENTS
The author is grateful to Professor Jan Löfqvist for providing funding for the project through the Swedish Agency for
Research Cooperation with Developing Countries (SAREC). Critical reading of the manuscript was done by several
colleagues in the pheromone group at Lund University. I am also grateful to Äsa Persson who aided in the bibliographical
research.
XI. REFERENCES
Adams, C. M. & Bernays, E. A. (1978). The effect of combinations of deterrents on the feeding behaviour of Locusta
migratoria. Entomologia experimentalis et applicata 23, 101-109.
Alam, S. M. (1952). Food plants of desert locust. Current Science 21, 344.
Ali, M.F. & Morgan, E.D. (1990). Chemical communication in insect communities: A guide to insect pheromones with special
emphasis on social insects. Biological Reviews 65, 227-247.
Ameismeier, F. (1987). Ultrastructure of the chemosensitive basiconic single-walled wall-pore sensilla on the antennae in
adults and embryonic stages of Locusta migratoria L. (Insecta, Orthoptera). Cell and Tissue Research
247, 605-612.
Amerasinghe, F. P. (1978a). Pheromonal effects on sexual maturation, yellowing, and the vibration reaction in immature male
desert locusts (Schistocerca gregaria). Journal of Insect Physiology 24, 309-314.
Amerasinghe, F. P. (1978b). Effects of J.H.I and J.H.III on yellowing, sexual activity and pheromone production in
allatectomized male Schistocerca gregaria. Journal of Insect Physiology 24, 603-611.
Ashall, C. & Ellis, P. E. (1961). Studies on numbers and mortality in field populations of the desert locust (Schistocerca
gregaria Forskål). Anti-Locust Bulletin 38, 1-59.
Ba-Angood, S. A. S. (1976). Preliminary observations on the effect of crowdness by grasshoppers on phase development of
the desert locust Schistocerca gregaria. Zeitschrift für angewandte Entomologie 80, 228-231.
Baker, P. S., Gewecke, M. & Cooter, R. J. (1984). Flight orientation of swarming Locusta migratoria. Physiological
Entomology 9, 247-252.
Baker, T.C. (1989). Sex pheromone communication in the Lepidoptera: New research progress. Experientia
45, 248-262.
Bennett, L. V. (1975). Development of a desert locust plague. Nature 256, 486-487.
Bernays, E. A. & Lee, J. C. (1988). Food aversion learning in the polyphagous grasshopper Schistocerca americana
Physiological Entomology 13, 131-137.
Bertram, B. C. R. (1978). Living in groups: predators and prey. In Behavioural Ecology. (ed. J. R. Krebs and N. B.
Davies), pp. 64-96. Blackwell Scientific Publications, London.
Bhatia, D. R. (1940). Observations on the biology of desert locust (Schistocerca gregaria F.) in Sind-Rajputana area.
Indian Journal of Entomology 2, 187-192.
Blaney, W. M. (1975). Behavioural and electrophysiological studies of taste discrimination by the maxillary palps of larvae of
Locusta migratoria (L). Journal of Experimental Biology 62, 555- 569.
Blaney, W. M. (1980). Chemoreception and food selection by locusts. In Olfaction and Taste (ed. H. van der Starre),
pp. 127-149. Information Retrieval Ltd. Press, London.
Blaney, W. M. & Simmonds, M. S. J. (1990). The chemoreceptors. In Biology of Grasshoppers. (ed. R. F. Chapman
and A. Joern), pp. 1-37. John Wiley and Sons, New York, USA.
Blight, M. M. (1969). Volatile nitrogenous bases emanating from laboratory-reared colonies of the desert locust,
Schistocerca gregaria. Journal of Insect Physiology 15, 259-272.
Brower, J. V. Z. (1960). Experimental studies of mimicry. IV. The reactions of starlings to different proportions of models and
mimics. American Naturalist 94, 271-282.
Burghardt, G. M. (1970). Defining "Communication". In Communication by Chemical Signals (ed. J.W. Johnston, Jr.,
D. G. Moulton & A. Turk), pp. 5-18. Appleton-Century-Crofts, New York.
Butterworth, J. H. & Morgan, E. D. (1971). Investigation of the locust feeding inhibition of the seeds of the neem tree
Azadirachta indica. Journal of Insect Physiology 17, 969-977.
Byers, J.A. (1988). Novel diffusion-dilution method for release of semiochemicals: Testing pheromone component ratios on
western pine beetle. Journal of Chemical Ecology 14, 199-212.
Byers, J.A. (1989). Chemical ecology of bark beetles. Experientia 45, 271-283.
Byers, J.A. (1991). Simulation of the mate-finding behaviour of pine shoot beetles, Tomicus piniperda. Animal
Behaviour 41, 649-660.
Byers, J.A., Birgersson, G., Löfqvist, J., Appelgren, M. & Bergström, G. (1990). Isolation of pheromone synergists of bark
beetle, Pityogenes chalcographus, from complex insect-plant odors by fractionation and subtractive-combination
bioassay. Journal of Chemical Ecology 16, 861-876.
Carlisle, D. B., Ellis, P. E. & Betts, E. (1965). The influence of aromatic shrubs on sexual maturation in the desert locust
Schistocerca gregaria. Journal of Insect Physiology 11, 1541- 1558.
Cassier, P. P. & Delorme-Joulie, C. (1976). The imaginal integumental differentiation in Schistocerca gregaria Forsk.
III. Phase polymorphism and its hormonal control. Insectes Sociaux 23, 179-198.
Cassier, P. & Papillon, M. (1983). Le dimorphism sexuel des protéines cuticularies du Criquet migrateur, Locusta
migratoria migratorioides (Reiche & Farmaire). Canadian Journal of Zoology 61, 2976-2986.
Cassier, P., Porcheron, P., Papillon, M. & Lensky, Y. (1980). Study of the cuticular proteins of the African migratory locust,
Locusta migratoria migratorioides (R. and F.). Quantitative data. Annales de Sciences Naturelles Zoologie
2, 51-65.
Chapman, R. F. (1974). The chemical inhibition of feeding by phytophagous insects: a review. Bulletin of Entomological
Research 64, 339-363.
Chapman, R. F. (1982). Chemoreception: The significance of receptor numbers. Advances in Insect Physiology
16, 247-365.
Charnley, A. K., Hunt, J. & Dillon, R. J. (1985). The germ-free culture of desert locusts, Schistocerca gregaria. Journal of
Insect Physiology 31, 477-485.
Cook, A. G. (1977). Nutrient chemicals as phago stimulants for Locusta migratoria. Ecological Entomology. 2,
113-122.
Dale, J. F. & Tobe, S. A. (1990). The endocrine basis of locust phase polymorphism. In Biology of Grasshoppers. (ed.
R. F. Chapman and A. Joern), pp. 393-414. John Wiley and Sons, New York, USA.
Davenport, A. P. & Evans, P. D. (1984). Stress-induced changes in the octopamine levels of insect haemolymph. Insect
Biochemistry 14, 135-143.
Davey, J. T. & Johnston, H. B. (1956). The African migratory locust (Locusta migratoria migratorioides R. & F.)
in Nigeria. Anti-Locust Bulletin 22, 1-91.
Dearn, J. M. (1974a). Phase transformation and chiasma frequency variation in locusts. I. Schistocerca gregaria.
Chromosoma 45, 321-338.
Dearn, J. M. (1974b). Phase transformation and chiasma frequency variation in locusts. II. Locusta migratoria.
Chromosoma 45, 339-352.
Dirsh, V. M. (1953). Morphometrical studies on phases of the desert locust (Schistocerca gregaria Forskål). Anti-
Locust Bulletin 16, 1-34.
Ellis, P. E. (1951). The marching behaviour of hoppers of the African migratory locust in the laboratory. Anti-Locust
Bulletin 7, 1-48.
Ellis, P. E. & Ashall, C. (1957). Field studies in diurnal behaviour, movement and aggregation in the desert locust
(Schistocerca gregaria Forskål). Anti-Locust Bulletin 25, 1-94.
Evans, C. S. & Bell, E. A. (1979). Nonprotein amino-acids of acacia species and their effect on the feeding of the acridids
Anacridium melanorhodon and Locusta migratoria. Phytochemistry. 18, 1807-1810.
Evans, P. D. (1985). Octopamine. In Comprehensive Insect Physiology, Biochemistry and Pharmacology. (eds. A. G.
Kerkut and L. I. Gilbert), Vol. 11, pp. 499-530. Pergamon Press, Oxford.
Farrow, R. A. (1990). Flight and migration in acridoids. In Biology of Grasshoppers. (ed. R. F. Chapman and A. Joern),
pp. 227-314. John Wiley and Sons, New York, USA.
Fuzeau-Braesch, S., Genin, E., Jullien, R., Knowles, E. & Papin, C. (1988). Composition and role of volatile substances in
atmosphere surrounding two gregarious locusts, Locusta migratoria and Schistocerca gregaria. Journal of Chemical
Ecology 14, 1023-1033.
Gill, J. S. & Lewis, C. T. (1971). Systemic action of an insect feeding deterrent. Nature 232, 402-403.
Gillett, S. D. (1968). Airborne factor affecting the grouping behaviour of locusts. Nature 218, 782-783.
Gillett, S. D. (1973). The role of integumental colour pattern in locust grouping. Animal Behaviour 21,
153-156.
Gillett, S. D. (1975a). Changes in the social behaviour of the desert locust, Schistocerca gregaria, in response to the
gregarizing pheromone. Animal Behaviour 23, 494-503.
Gillett, S. D. (1975b). The action of the gregarisation pheromone on five non-behavioural characters of phase polymorphism
of the desert locust, Schistocerca gregaria (Forskål). Acrida 4, 137-150.
Gillett, S. D. (1983). Primer pheromones and polymorphism in the desert locust. Animal Behaviour 31,
221-230.
Gillett, S. D. (1988). Solitarization in the desert locust, Schistocerca gregaria (Forskål) (Orthoptera: Acrididae).
Bulletin of Entomological Research 78, 623-631.
Gillett, S. D., Packham, J. M. & Papworth, S. J. (1976). Possible pheromonal effects on aggregation and dispersion in the
desert locust, Schistocerca gregaria (Forsk.). Acrida 5, 287- 297.
Gillett, S. D. & Phillips, M. L. (1977). Faeces as a source of a locust gregarisation stimulus. Effects on social aggregation and
on cuticular colour of nymphs of the desert locust, Schistocerca gregaria (Forsk.). Acrida 6,
279-286.
Grainge, M. & Ahmed, S. (1988). Handbook of Plants with Pest-Control Properties. John Wiley and Sons, New York.
Greenwood, M. & Chapman, R. F. (1984). Differences in numbers of sensilla on the antennae of solitarious and
gregarious Locusta migratoria L. (Orthoptera: Acrididae). International Journal of Insect Morphology and
Embryology 13, 295-301.
Guichard, K. M. (1955). Habitats of the desert locust (Schistocerca gregaria Forskål) in western Libya and Tibesti.
Anti-Locust Bulletin 21, 1-33.
Hamilton, W. J. (1967). Social aspects of bird orientation mechanisms. In Animal Orientation and Navigation (ed. R. M.
Storm), pp. 57-71. Oregon State University Press, Corvallis.
Harjai, S. C. (1974). Study of a pocket of the desert locust population in Rajasthan, with special reference to biometrics and
behaviour. Indian Journal of Entomology 36, 329-343.
Harjai, S. C. & Sikka, H. L. (1970). Effect of soil moisture on the phase characters of hatchlings in the desert locust,
Schistocerca gregaria Forsk. Indian Journal of Entomology 32, 298-302.
Haskell, P. T. (1957) The influence of flight noise on behaviour in the desert locust Schistocerca gregaria (Forsk.).
Journal of Insect Physiology 1, 52-75.
Haskell, P. T. & Schoonhoven, L. M. (1968). The function of certain mouth part receptors in relation to feeding in
Schistocerca gregaria and Locusta migratoria migratorioides. Entomologia Experimentalis et Applicata
12, 423-440.
Haskell, P. T., Paskin, M. W. J. & Moorhouse, J. E. (1962). Laboratory observations on factors affecting the movements of
hoppers of the desert locust. Journal of Insect Physiology 8, 53-78.
Highnam, K. C. & Haskell, P. T. (1964). The endocrine systems of isolated and crowded Locusta and
Schistocerca in relation to oöcyte growth, and the effects of flying upon maturation. Journal of Insect
Physiology 10, 849-864.
Highnam, K. C. & Lusis, O. (1962). The influence of mature males on the neurosecretory control of ovarian development in
the desert locust. Quarterly Journal of Microscopical Science 103, 73-81.
Hunter-Jones, P. (1962). Coloration of the desert locust (Schistocerca gregaria Forsk.) reared in isolation.
Entomological monthly Magazine 98, 89-93.
Hurter, J., Boller, E. F., Städler, E., Blattmann, B., Buser, H.R., Bosshard, N.U., Damn, L., Kozolwski, M.W., Schöni, R.,
Raschdorf, F., Schlumpf, E., Fritz, H., Richter, W.J. & Schreiber, J. (1987). Oviposition-deterring pheromone in Rhagoletis
cerasi L.: Purification and determination of the chemical constitution. Experientia 45, 157-164.
Husain, M. A., Mathur, C. B. & Roonwal, M. L. (1946). Studies on Schistocerca gregaria (Forskål). XIII. Food and
feeding habit of desert locusts. Indian Journal of Entomology 8, 141- 163.
Johnston, H. B. & Buxton, D. R. (1949). Field observations on locusts in eastern Africa. Anti- Locust Bulletin
5, 1-74.
Joyce, R. J. V. (1952). The ecology of grasshoppers in east central Sudan. Anti-Locust Bulletin 11, 1-
99.
Kafka, W. A., Ohloff, G., Schneider, D. & Vareschi, E. (1973). Olfactory discrimination of 2 enantiomers of 4 methyl hexanoic-
acid by the migratory locust and the honey bee. Journal of Comparative Physiology 87, 277-284.
Kendall, M. D. (1972). Glandular epidermis on the tarsi of the desert locust, Schistocerca gregaria Forskål.
Acrida 1, 121-147.
Kennedy, J. S. (1962). The division of labour between the phases of locusts. Colloques Internationaux du Centre national
de la Recherche Scientifiques 114, 269-297.
Kennedy, J. S. & Moorhouse, J. E. (1969). Laboratory observations on locust responses to wind- borne grass odour.
Entomologia experimentalis et applicata 12, 487-503.
Khan, A. A. (1979). Mortality of desert locust eggs in the laboratory by a nematode. Pakistani Journal of Zoology
11, 51-55.
Lange, A. B. & Loughton, B. G. (1985). An oviposition-stimulating factor in the male accessory reproductive gland of the
locust, Locusta migratoria. General and Comparative Endocrinology 57, 208-215.
Lauga, J. & Hatte, M. (1977). Acquisition de proprietes gregarisantes par le sable utilise a la ponte repetee de femelles
gregaires de Locusta migratoria L. (Ins. Orthop.). Acrida 6, 307-311.
Lauga, J. & Hatte, M. (1978). L'activite gregarisante du sable de ponte chez Locusta migratoria L.: action sur le
comportement et la reproduction des individus. Annales des Sciences Naturelles (Zoologie) 20, 3752.
Lea, A. (1962). The nature and significance of phase variation in the brown locust Locustana pardalina (Walk.).
Colloques Internationaux du Centre national de la Recherche Scientifiques 114, 241-255.
Leahy, M. G. (1973). Oviposition of virgin Schistocerca gregaria (Forskål) (Orthoptera: Acrididae) after implant of the
male accessory gland complex. Journal of Entomology (A) 48, 69-78.
Lee, J. C. & Bernays, E. A. (1990). Food tastes and toxic effects: associative learning by the polyphagous grasshopper
Schistocerca americana (Drury)(Orthoptera: Acrididae). Animal Behaviour 39, 163-173.
Loher, W. (1958). An olfactory response of immature adults of the desert locust. Nature 181, 1280.
Loher, W. (1960). The chemical acceleration of the maturation process and its hormonal control in the male of the desert
locust. Proceedings of the Royal Society of London (B) 153, 380-397.
Loher, W. (1990). Pheromones and phase transformation in locusts. In Biology of Grasshoppers. (ed. R. F. Chapman
and A. Joern), pp. 337-355. John Wiley and Sons, New York, USA.
Mann, H. H. & Burns, W. (1927). Locust attack of 1926-27 in Sind, Kathiawar and Gujrat. Agricultural Journal of India
22, 325-326.
Michel, R. (1971). Influence du groupement en essaim artificiel sur la tendance au vol du criquet pélerin (Schistocerca
gregaria Forsk). Behaviour 39, 58-72.
Michel, R. (1980). Development of flight behaviour of successive generations of desert locust (Schistocerca gregaria)
raised in isolation then in groups. Animal Behaviour 28, 1288-1289.
Monache, F. D., Bettolo, G. B. M. & Bernays, E. A. (1984). Isolation of insect antifeedant alkaloids from Maytenus
rigida (Celastraceae). Zeitschrift für angewandte Entomologie 97, 406- 414.
Mordue, A. J. (1977). Some effects of amputation of the antennae on pigmentation, growth and development in the locust,
Schistocerca gregaria. Physiological Entomology. 2, 293- 300.
Mwangi, R. W. (1982). Locust antifeedant activity in fruits of Melia volkensii. Entomologia experimentalis et
applicata 32, 277-280.
Navon, A. & Bernays, E. A. (1978). Inhibition of feeding in acridids by non-protein amino acids. Comparative Biochemistry
and Physiology. A. Comparative Physiology 59, 161-164.
Nickerson, B. (1956). Pigmentation of hoppers of the desert locust (Schistocerca gregaria Forskål) in relation to phase
coloration. Anti-Locust Bulletin 24, 1-34.
Nolte, D. J. (1963). A pheromone for melanization of locusts. Nature 200, 660-661.
Nolte, D. J. (1967). Phase transformation and chiasma formation in locusts. Chromosoma 21, 123- 139.
Nolte, D. J. (1968). The chiasma-inducing pheromone of locusts. Chromosoma 23, 346-358.
Nolte, D. J. (1969). Chiasma-induction and tyrosinase metabolism in locusts. Chromosoma 26, 287-297.
Nolte, D. J. (1973). The relationship of locust species to locustol. Acrida 2, 53-60.
Nolte, D. J. (1974). The gregarization of locusts. Biological Reviews 49, 1-14.
Nolte, D. J. (1976). Locustol and its analogues. Journal of Insect Physiology 22, 833-838.
Nolte, D. J. (1977). The action of locustol. Journal of Insect Physiology 23, 899-903.
Nolte, D. J., Eggers, S. H. & May, I. R. (1973). A locust pheromone: Locustol. Journal of Insect Physiology
19, 1547-1554.
Nolte, D. J., May, I. R. & Thomas, B. M. (1970). The gregarisation pheromone of locusts. Chromosoma 29,
462-473.
Norris, M. J. (1954). Sexual maturation in the desert locust (Schistocerca gregaria Forskål) with special reference to
the effects of grouping. Anti-Locust Bulletin 18, 1-44.
Norris, M. J. (1962). Group effects on the activity and behaviour of adult males of the desert locust (Schistocerca
gregaria Forsk.) in relation to sexual maturation. Animal Behaviour 10, 275-291.
Norris, M. J. (1963). Laboratory experiments on gregarious behaviour in ovipositing females of the desert locust,
(Schistocerca gregaria (Forsk.)). Entomologia experimentalis et applicata 6, 279-303.
Norris, M. J. (1964). Accelerating and inhibiting effects of crowding on sexual maturation in two species of locusts.
Nature 203, 784-785.
Norris, M. J. (1970). Aggregation response in ovipositing females of the desert locust, with special reference to the chemical
factor. Journal of Insect Physiology 16, 1493-1515.
Odhiambo, T. R. (1966). Growth and the hormonal control of sexual maturation in the male desert locust, Schistocerca
gregaria (Forskål). Transactions of the Royal Entomological Society of London 118, 393-412.
Odhiambo, T. R. (1969). The architecture of the accessory reproductive glands of the male desert locust 1: Types of glands
and their secretions. Tissue and Cell 1, 155-182.
Ohabuike, J. E. (1979). Grass availability and food preference of the African migratory locust, Locusta migratoria
migratorioides. Zeitschrift für angewandte Entomologie 88, 354-363.
Okelo, O. (1971). Physiological control of oviposition in the female desert locust Schistocerca gregaria. Canadian Journal
of Zoology 49, 969-974.
Pener, M. P. (1965). On the influence of corpora allata on maturation and sexual behaviour of Schistocerca gregaria.
Journal of Zoology (Lond.) 147, 119-136.
Pener, M. P. (1967a). Effects of allatectomy and sectioning of the nerves of the corpora allata on oocyte growth, male sexual
behaviour and colour change in adults of Schistocerca gregaria. Journal of Insect Physiology 13, 665-
684.
Pener, M. P. (1967b). Comparative studies on reciprocal interchange of corpora allata between males and females of adult
Schistocerca gregaria (Forskål) (Orthoptera: Acrididae). Proceedings of the Royal Entomological Society of London
(A) 42, 139-148.
Peschke, K. (1987). Cuticular hydrocarbons regulate mate recognition, male aggression, and female choice of the rove
beetle, Aleochara curtula. Journal of Chemical Ecology 13, 1993-2008.
Pickford, R., Ewen, A. B. & Gillott, C. (1969). Male accessory gland substance: An egg-laying stimulant in Melanoplus
sanguinipes (F). Canadian Journal of Zoology 47, 1199- 1203.
Popov, G. B. (1953). Investigations of suspected outbreak areas of the desert locust (Schistocerca gregaria Forskål) in
Iran. Anti-Locust Bulletin 14, 1-32.
Popov, G. B. (1958). Ecological studies on oviposition by swarms of the desert locust (Schistocerca gregaria Forskål)
in eastern Africa. Anti-Locust Bulletin 31, 1-70.
Pradhan, S., Jotwani, M. G. & Rai, B. K. (1962). The neem seed deterrent to locust. Indian Farming 12, 7-
11.
Prokopy, R. J. (1981). Epideictic pheromones that influence spacing patterns of phytophagous insects. In
Semiochemicals: Their Role in Pest Control (ed. D. A. Nordlund, R. L. Jones and W. J. Lewis), pp. 181-213. John
Wiley and Sons, New York, USA.
Rainey, R. C. (1989). Migration and Meteorology: Flight Behaviour and the Atmospheric Environment of Locusts and Other
Migrant Pests. Clarendon Press, Oxford, UK.
Rainey, R. C. & Sayer, H. J. (1953). Some recent developments in the use of aircraft against flying locust swarms.
Nature 172, 224-228.
Rao, P. J. (1982). Phagostimulants and antifeedants from Calotropis gigantea for Schistocerca gregaria
Forskal: Distribution in different parts of the plant. Zeitschrift für angewandte Entomologie 93, 141-146.
Rao, P.J. & Mehrotra, K. N. (1977). Phagostimulants and antifeedants from Calotropis gigantea for Schistocerca
gregaria F. Indian Journal of Experimental Biology 15, 148-150.
Riley, J. R. (1975). Collective orientation in night-flying insects. Nature 253, 113-114.
Riley, J. R. & Reynolds, D. R. (1983). A long-range migration of grasshoppers observed in the sahelian zone of Mali by two
radars. Journal of Animal Ecology 52, 167-183.
Roffey, J. & Popov, G. (1968). Environmental and behavioural processes in a desert locust outbreak. Nature
219, 446-450.
Shurney, G. A. & Zottola, E. A. (1976). Biblical food processing. Journal of Milk and Food Technology 39,
439-441.
Siddiqi. J. I. & Khan, M. A. (1981). The secretion and perception of a sex pheromone in the grasshopper Hieroglyphus
nigrorepletus Bolivar (Orthoptera: Acrididae). Acrida 10, 233-239.
Silverstein, R. M. (1981). Pheromones: Background and potential for use in insect pest control. Science 213, 1326-
1332.
Simpson, S. J., Simmonds, M. S. J. & Blaney, W. M. (1988). A comparison of dietary selection behaviour in larval Locusta
migratoria and Spodoptera littoralis. Physiological Entomology. 13, 225-238.
Singh, R. P. (1983). Search for antifeedants in some botanicals for desert locust, Schistocerca gregaria Forskal.
Zeitschrift für angewandte Entomologie 96, 316-319.
Singh, R. P. & Pant, N. C. (1980). Lycorine-a resistance factor in the plants of subfamily Amaryllidoideae (Amaryllidaceae)
against desert locust, Schistocerca gregaria F. Experientia 36, 552-553.
Singh, C. & Singh, J. (1977). Rann of Kutch - a trap for the locusts. Indian Journal of Entomology 39,
23-28.
Stower, W. J. & Greathead, D. J. (1969). Numerical changes in a population of the desert locust with special reference to
factors responsible for mortality. Journal of Applied Ecology 6, 203- 235.
Stower, W. J., Popov, G. B. & Greathead, D. J. (1958). Oviposition behaviour and egg mortality of the desert locust
(Schistocerca gregaria Forskål) on the coast of Eritrea. Anti-Locust Bulletin 30, 1-33.
Strong, L. (1970). Epidermis and pheromone production in males of the desert locust. Nature 228,
285-286.
Strong, L. (1971). Intracellular ducts in the epidermis of the male desert locust. Journal of Insect Physiology
17, 1823-1831.
Strong, L. & Amerasinghe, F. P. (1977). Allatectomy and sexual receptivity in females of Schistocerca gregaria. Journal of
Insect Physiology 23, 131-135.
Szentesi, A. & Bernays, E. A. (1984). A study of behavioural habituation to a feeding deterrent in nymphs of Schistocerca
gregaria. Physiological Entomology 9, 329-340.
Thomas, J. G. (1965). The abdomen of the female desert locust (Schistocerca gregaria Forskål) with special reference
to the sense organs. Anti-Locust Bulletin 42, 1-22.
Thomas, J. G. (1966). The sense organs on the mouthparts of the desert locust Schistocerca gregaria. Journal of
Zoology 148, 420-448.
Thomas, J. G. (1970). Probable pheromone-secreting cells in the epidermis of mature males of Schistocerca gregaria
Forskål (Orthoptera: Acrididae). Proceedings of the Royal Entomological Society of London (A) 45, 125-
135.
Uvarov, B. P. (1921). A revision of the genus Locusta L. (Pacytylus L Fieb.), with a new theory as to the
periodicity and migrations of locusts. Bulletin of Entomological Research 12, 135-163.
Wallraff, H. G. (1978). Social interrelations involved in migratory orientation of birds: possible contribution of field studies.
Oikos 30, 401-404.
Waloff, Z. (1972). Orientation of flying locusts, Schistocerca gregaria (Forsk.), in migrating swarms. Bulletin of
Entomological Research 62, 1-72.
Waloff, Z. & Green S. M. (1975). Regularities in duration of regional desert locust plagues. Nature 256, 484-
485.
White, P. R. & Chapman, R. F. (1990). Tarsal chemoreception in the polyphagous grasshopper Schistocerca
americana: behavioural assays, sensilla distributions and electrophysiology. Physiological Entomology
15, 105-121.
Whitman, D. W. (1982). Grasshopper sexual pheromone: A component of the defensive secretion in Taeniopoda
eques. Physiological Entomology 7, 111-115.
Whitman, D. W. (1990). Grasshopper chemical communication. In Biology of Grasshoppers. (ed. R. F. Chapman and
A. Joern), pp. 357-391. John Wiley and Sons, New York, USA.
Winstanley, C. & Blaney, W. M. (1978). Chemosensory mechanisms of locusts in relation to feeding. Entomologia
experimentalis et applicata 24, 550-558.
Worm, R. A. A. (1980). Involvement of cyclic nucleotides in locust flight muscle metabolism. Comparative Biochemistry
and Physiology C 67, 23-27.
Yinon, U., Shulov, A. & Tsvilich, R. (1971). Audition in the desert locust: Behavioural and neurophysiological studies.
Journal of Experimental Biology 55, 713-725.


 (a) Isolation of pheromone
(a) Isolation of pheromone (a) Isolation of pheromone
(a) Isolation of pheromone
