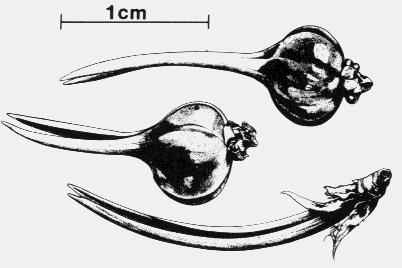
 Round galls (redish colored at base) at tips of branches of pinyon pine tree.
Round galls (redish colored at base) at tips of branches of pinyon pine tree.
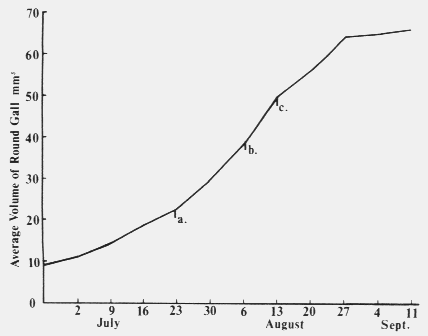
 to the laboratory where the normal needles
and galls containing the larvae were frozen
in liquid nitrogen then each crushed separately
with dry ice to a powder-like consistency using
a mortar and pestle. The material subsequently
was lyophilized and stored at -20oC until
needed.
to the laboratory where the normal needles
and galls containing the larvae were frozen
in liquid nitrogen then each crushed separately
with dry ice to a powder-like consistency using
a mortar and pestle. The material subsequently
was lyophilized and stored at -20oC until
needed.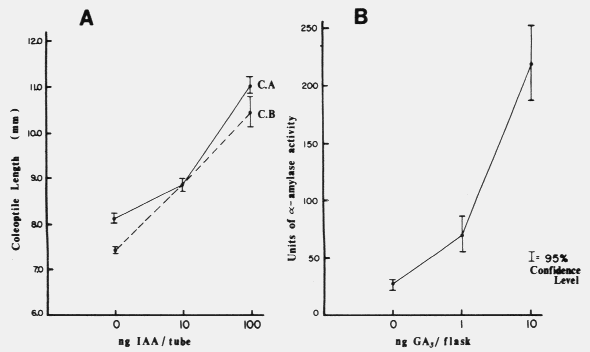
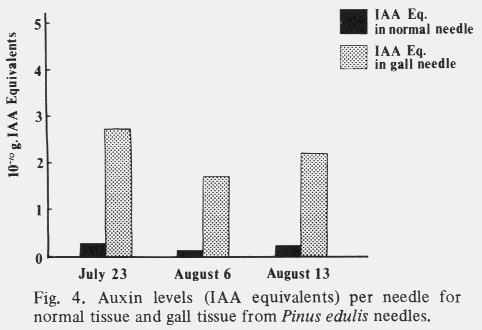
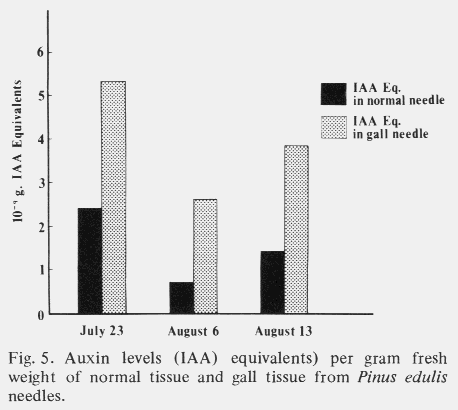
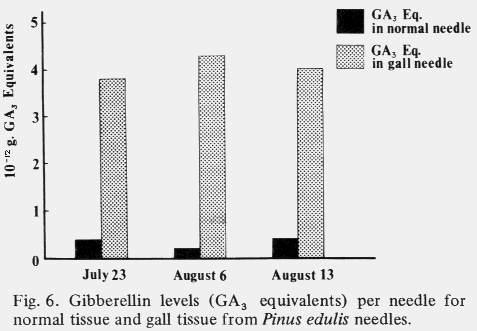
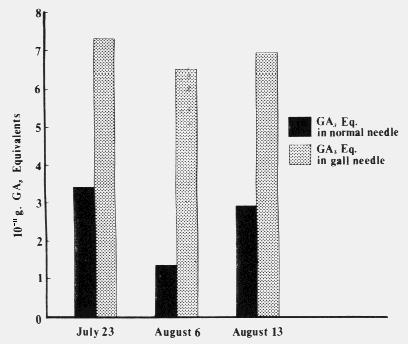
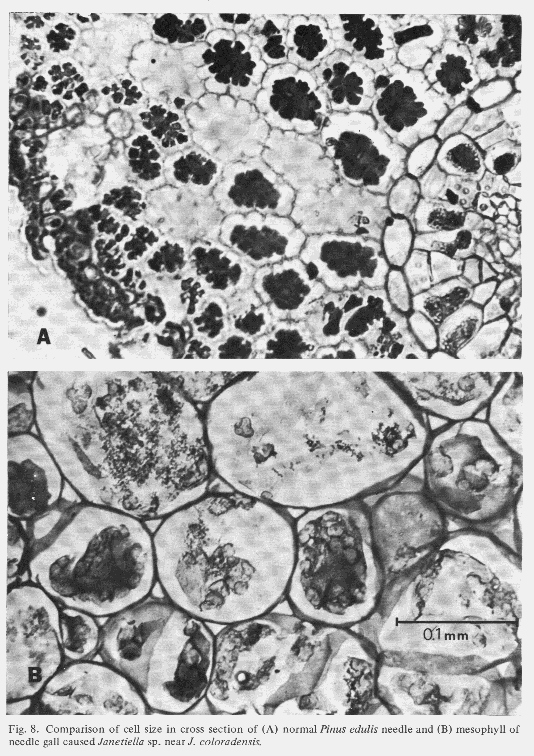
| Table 1. Comparison of mean cell size and number in cross sections through the middle of normal and galled needles of the same age. (Aug. 1,1972) | ||
| Normal needle | Galled needle | |
|---|---|---|
| No. epidermal cells | 151 | 300 |
| No. cells inside endodermis | 402 | 501 |
| No. endodermal cells | 27 | not recognizable |
| No. cells outside endodermis | 558 | 1,527 |
| No. cells total | 987 | 2,028 |
| Area outside endodermis (mm)2 | 0.54 | 8.29 |
| Diameter of average cell outside endodermis (mm) | 3.25 x 10-2 | 8.31 x 10-2 |
| Volume of average cell outside endodermis (mm)3 | 2.30 x 10-5 | 30.10 x 10-5 |
| X-fold increase. in volume of average cell outside endodermis | 1 | 13.1 |