1Department of Animal Ecology, Lund University, SE-223 62 Lund, Sweden
Present address:

2Chemical Ecology, Göteborg University, SE-405 30 Göteborg, Sweden
 Set of pipe traps with funnel (used in this paper) in Scotch pine forest in southern Sweden.
Set of pipe traps with funnel (used in this paper) in Scotch pine forest in southern Sweden.
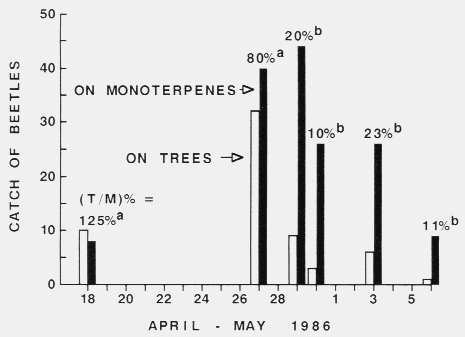 Figure 1. Relative attraction rates of Tomicus piniperda to six winter-storm-fallen
Scots pine trees with twenty flight-barrier traps and to constant release rates
of monoterpenes from four perforated cylinder traps
during the annual spring colonization period (Sjöbo, southern Sweden).
The percentage of catch (based on total for a trap type; trees; monoterpenes = T/M %)
between dates were significantly different (alpha = 0.05, x2) if
designated with different letters. Each cylinder traps released (per day) a
mixture of monoterpenes (14mg each (+)-a-pinene, < 99.5 % pure,
[alpha]20546 = + 57o, and (-)-a-pinene. < 99.5 %,
[alpha]20546, = - 50o; 6 mg (+)-3-carene,
< 99 %, [alpha]20D = + 17o, all Fluka; and 2.5 mg
terpinolene, < 97.3 %, Carl Roth) as described in the table.
Figure 1. Relative attraction rates of Tomicus piniperda to six winter-storm-fallen
Scots pine trees with twenty flight-barrier traps and to constant release rates
of monoterpenes from four perforated cylinder traps
during the annual spring colonization period (Sjöbo, southern Sweden).
The percentage of catch (based on total for a trap type; trees; monoterpenes = T/M %)
between dates were significantly different (alpha = 0.05, x2) if
designated with different letters. Each cylinder traps released (per day) a
mixture of monoterpenes (14mg each (+)-a-pinene, < 99.5 % pure,
[alpha]20546 = + 57o, and (-)-a-pinene. < 99.5 %,
[alpha]20546, = - 50o; 6 mg (+)-3-carene,
< 99 %, [alpha]20D = + 17o, all Fluka; and 2.5 mg
terpinolene, < 97.3 %, Carl Roth) as described in the table.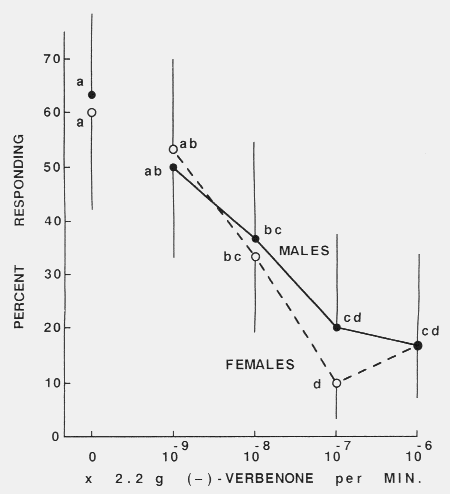 Figure 2. Effect of increasing release rates of (-)-verbenone on the attraction response
of walking male and female Tomicus piniperdu to a
1:1:1:1 mixture of host monoterpenes (-)-a-pinene, (+)-a-pinene, (+)-3-carene
and terpinolene (chemical purity as in fig.1, table) each released
at 2.2 x 10-6 g/min. Points (N = 30) with the same letters wcre not significantly
different (alpha = 0.05, x2). The vertical lines reprcsent upper or lower
95 % confidence limits for proportions.
Figure 2. Effect of increasing release rates of (-)-verbenone on the attraction response
of walking male and female Tomicus piniperdu to a
1:1:1:1 mixture of host monoterpenes (-)-a-pinene, (+)-a-pinene, (+)-3-carene
and terpinolene (chemical purity as in fig.1, table) each released
at 2.2 x 10-6 g/min. Points (N = 30) with the same letters wcre not significantly
different (alpha = 0.05, x2). The vertical lines reprcsent upper or lower
95 % confidence limits for proportions.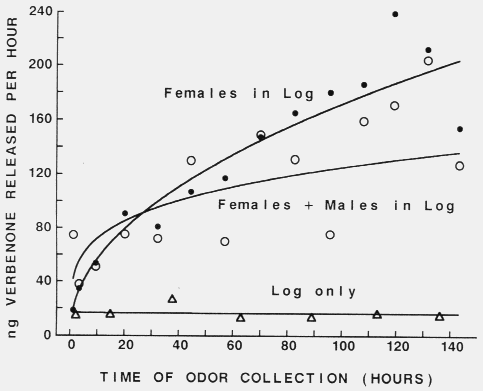 Figure 3. Release of verbenone from Scots pine logs infested with 50
female (filled circles), or 50 female plus 50 male (open circles) Tomicus piniperda,
or not infested but drilled with 50 holes (triangles). Lines were
fitted with geometric curve regression, Y = 18.93X0.479 (females in log),
Y = 41.73X0.238 (females + males in log) and Y = 16.94X-0.008 (log
only).
Figure 3. Release of verbenone from Scots pine logs infested with 50
female (filled circles), or 50 female plus 50 male (open circles) Tomicus piniperda,
or not infested but drilled with 50 holes (triangles). Lines were
fitted with geometric curve regression, Y = 18.93X0.479 (females in log),
Y = 41.73X0.238 (females + males in log) and Y = 16.94X-0.008 (log
only).| Attraction of Tomicus piniperda to perforated cylinder traps" releasing attractive host monoterpenes and inhibitory enantiomers of verbenone (V) in Scots pine forest, Sjöbo, southern Sweden. T. piniperda beetles were caught in the outer funnel (while landing) and only one entered a hole in the perforated cylinder. | ||
| Chemicals releaseda | Total catch of cylindar traps | |
|---|---|---|
| Males | Females | |
| 14 - 20 May 1985 (9 replicates) | ||
| Blank | 0 | 0 |
| Monoterpenesb | 30 | 29 |
| Monoterpenes + (+)-V | 9 | 3 |
| Monoterpenes + (-)-V | 0 | 3 |
| 17 - 29 April 1986 (13 replicates) | ||
| Blank | 1 | 0 |
| Monoterpenesb | 83 | 65 |
| Monoterpenes + (+)-V | 35 | 16 |
| Monoterpenes + (-)-V | 19 | 19 |
|
J. A. BYERS1, B. S. LANNE2, J. LÖFQVIST1 1Department of Animal Ecology, Lund University, SE-223 62 Lund, Sweden Present address: 
2Chemical Ecology, Göteborg University, SE-405 30 Göteborg, Sweden |
|---|