Byers, J.A. 1989. Behavioral mechanisms involved in reducing competition in bark
beetles. Holarctic Ecology 12: 466-476.  pdf
pdf
 Bark beetles such as Ips typographus
at the top of the Norway spruce log, Picea abies, must compete for the relatively thin layer of bark-phloem
as seen in the brown area around the log.
Bark beetles such as Ips typographus
at the top of the Norway spruce log, Picea abies, must compete for the relatively thin layer of bark-phloem
as seen in the brown area around the log.
ABSTRACT
Bark beetles feed and reproduce in the
phloem/cambium tissue of trees where severe competition between individuals of the
same and different species significantly re- duces their reproductive success. In this
coevolutionary setting, individuals that can avoid competition whenever possible by
means of genetically controlled behavioral mechanisms are naturally selected.
Avoidance of intraspecific and interspecific com- petition is accomplished in part even
before landing by olfactory perception of specific pheromones and allomones. These
olfactory systems may function at least as well after landing. In several species the
uniform distribution of attacks on host trees indicates that individuals avoid competition
by only attacking if they are at least a minimum distance from other established
attacks. Once beetles are under the bark their tunneling patterns indicate that a
behavioral mechanism exists to avoid intersecting nearby galleries of competing
individuals. Finally, beetles may choose to re-emerge if the expected chances of
successful reproduction by continuing their stay become less than the probabilities of
finding another host and successfully reproducing.
Introduction "Aggressive" bark
beetles (Coleoptera: Scolytidae), those that often kill a tree in a "mass attack" in order
to reproduce, are especially damaging to coniferous forests. A discussion and review
of literature and concepts concerning bark beetle competition for host-tree resources
and the effects of interactions of host resistance and beetle virulence/cooperation on
such competition may elucidate new approaches to research programs with the
ultimate goal of integrated control of pest bark beetles. Two important bark beetle
species of California, Dendroctonus brevicomis and Ips paraconfusus,
will be compared with I. typographus of Europe concerning the state of our
knowledge about the behavioral processes which may operate to reduce competition.
Research on closely related species will be discussed to illustrate hypotheses and
possible behavioral mechanisms when our knowledge of the above bark beetles is
largely lacking.

Ips typographus
Host-tree resistance to bark beetles
Bark beetles usually confine their activities to the nutritious, but thin, layers of phloem
and cambium which are sandwiched between the outer bark and the often resinous
xylem tissue. The combined thickness of these layers is similar to the size of a beetle,
and competition can be quite severe for this two-dimensionally limited food resource.
Since the adults and their larvae consume the food transporting tissue, this would
result in the death of the tree. However, trees generally die first because symbiotic
fungi introduced by the beetle paralyze the water transporting system. The loss of
turgor pressure reduces resinosis and benefits the beetle (Wood 1982). Trees,
therefore, must expend considerable energy to defend themselves against bark beetle
attack and colonization.
Besides a tough and thick bark, many trees produce oleoresin as a resistance
mechanism. Oleoresin is believed to adversely affect bark beetles through both
chemical and physical means (Smith 1965, Hodges et al. 1979, Wood 1982, Byers et
al. 1984). Although there is some question as to the importance of various chemical
constituents in the tree for resistance to bark beetles (Byers 1981a, Raffa and
Berryman 1983) it is very probable that certain tree terpenoids, tannins and phenolics
are repellent and damaging to bark beetles as they are to many phytophagous insects
(Rosenthal and Janzen 1979). It is evident that bark beetles must mobilize
considerable energy reserves to detoxify these secondary plant substances and this
must contribute to the "stress level" and mortality of beetles. It has also been
commonly observed that resinous exudations make it more difficult for beetles to
excavate tunnels and that crystallization of the resin will entrap adults (Miller and Keen
1960, Byers et al. 1984). Thus, there has been a natural selection for aggressive bark
beetles that can partially compensate for the chemical and physical resistance
mechanisms of conifers.
On the other side, there is some reason to believe that mechanisms for resistance
to insects are selected for in trees. Sturgeon (1979) found correlative evidence that
in areas of high western pine beetle "predation" there were relatively more ponderosa
pine with higher proportions of a toxic monoterpene (limonene) than in other areas
without selection pressure. It was concluded that certain "resistant" high-limonene
trees may have been selected for as a result of high bark beetle-induced mortality.
Cooperation among bark beetles
In the coevolutionary "war" between pest bark beetles and their host trees it appears
that many species have responded to resinosis by utilizing pheromones which elicit
mass aggregations to overpower the tree (Wood 1982). Thus a minimum number or
density of beetles is required to cooperate in killing a particular tree and allow
reproduction. It is in the interests of each resident adult to advertise the suitability of
the food resource in order to obtain help in overcoming the tree's resistance. It is also
in the interests of each responding adult to join the attack providing it can determine
that successful colonization and brood production will result. The simple presence of
pheromone may indicate to arriving adults that the host tree has not been able to
resist attack, feeding, and release of pheromone and thus should provide suitable
breeding habitat. Several species in the genera Ips, Dendroctonus, and
Pityogenes are known to produce pheromone components only after feeding
(Coster and Vite 1972, Byers 1983a, Birgersson et al. 1984, Byers et al. 1988) which
is not possible while beetles must struggle against the copious flow of resin from a
resistant tree. Once the tree has been killed by the aggregated beetles, each individual
must contend or compete with the other beetles in the partitioning of the hark area.
Competition among bark beetles
At some point the attack density exceeds a certain value based on the characteristics
of the particular tree and bark beetle species, above which further increases in density
result in increasingly detrimental effects on reproduction due to intraspecific
competition between larvae (Berryman 1974). The nature of this competition is
probably a combination of interference competition (direct effects such as small larvae
being eaten) and exploitative competition (indirect effects such as food starvation).
Reports of intraspecific competition in several bark beetle species in the genera
Ips, Dendroctonus, Scolytus, and Tomicus have shown that brood output
per female decreases at higher attack densities (Miller and Keen 1960, McMullen and
Atkins 1961, Eidmann and Nuorteva 1968, Svihra 1972, Ogibin 1973, Beaver 1974,
Berryman 1974, Mayyasi et al. 1976, Wagner et al.1981, Light et al. 1983, Anderbrant
et al. 1985).
Interspecific competition has rarely been studied between scolytid species, but it is
hypothesized that resource partitioning (selection of specific host-tree species and/or
parts of a host) functions as a means of reducing competition between bark beetle
species in northwestern U.S.A. (Schmitz and Rudinsky 1968), California (Miller and
Keen 1960, Byers and Wood 1980) and in the southeastern U.S.A. (Paine et al. 1981).
The only laboratory study of interspecific competition has involved I.
paraconfusus and I. pini and showed severe effects of competition on
reproduction when densities were high (Light et al. 1983). Recent laboratory studies
with I. typographus and I. duplicatus (O. Anderbrant and F. Schlyter
unpubl.) and I. typographus and Pityogenes chalcographus (J. A. Byers
unpubl.) also show detrimental interspecific competition.
The adverse effects of competition become apparent even at relatively low densities
when beetles colonize wind-thrown trees and broken tops that are less able to resist
beetles (Raffa and Berryman 1983). However, the reproductive costs due to increased
crowding and competition which may determine whether a beetle decides to continue
the attack are probably balanced (in behavioral and evolutionary terms) by the costs
of searching elsewhere for suitable breeding areas.
The strong intra- as well as interspecific competition among bark beetles when
colonizing a host tree most probably have selected individuals adept at both avoiding
and surviving such competition. Behavioral mechanisms for avoiding competition may
operate in at least five ways: (1) avoid attraction to and landing in areas with high
attack density, (2) leave these areas after landing, (3) avoid initiating an attack near
others, (4) avoid tunneling into other gallery systems, and (5) re-emerge if densities
under the bark are too high. Interspecific competition can be avoided in addition to the
above ways by habitat/host selection (resource partioning, Paine et al. 1981, Byers and Wood 1980). Olfaction is known to be used in (1) and
(2) above while beetles may also use other sensory systems such as
auditory/stridulatory, thigmotactic, gustatory, and visual to avoid competition. Various
sensory systems may operate in several of the basic ways above but little is known
about mechanisms other than olfaction.
Olfactory mechanisms for avoiding intraspecific competition
If a beetle could determine while flying which areas on a tree were densely colonized
it would be advantageous because less time and energy would be required compared
with walking to gain the same information. Furthermore, flying beetles are relatively
immune to predation, especially by bark surface-hunting elerids and other predators.
Because of this, apparently, two of the most important pest bark beetles of California
on ponderosa pine Pinus ponderosa have evolved olfactory systems using
pheromones to avoid areas of higher attack densities.
In the California five-spined engraver I. paraconfusus, males initiate the
attack and release three synergistic components, (-)-ipsenol, (+)-ipsdienol and
(-)-(4S)-cis-verbenol, which are attractive to both sexes (Silverstein et al. 1966, Wood
et al. 1968). During feeding, males hydroxylate the host monoterpenes, myrcene and
(-)-a-pinene to the respective pheromone components (Hughes 1974, Renwick et al.
1976, Byers et al. 1979, Hendry et al. 1980, Byers 1983a). At lower concentrations of
the attractive pheromone (farther from the source) both sexes are about equally
attracted, but as the concentration increases, proportionately more males than females
do not fly directly to the source (Byers 1983b). In the laboratory bioassay it was shown
that at the higher release rates of pheromone components (2.2 x 10-7
and 2.2 x 10-6), the attraction response of walking males was inhibited
or reduced compared with lower rates, while female response increased proportional
to the dosage (Byers 1983b). During the attack of a felled ponderosa pine by I.
paraconfusus, relatively more males than females were found landing adjacent to the
origin of attack (baited with an infested log at the tree-top) while more females than
males were attracted to the origin. This pattern of males preceding females as
colonization pro- ceded down the trunk was evident for a few days until attacks were
occurring throughout the bole (Byers 1983b). Then at the peak of female aggregation
relatively fewer males landed on the tree and instead may have decided to search for
less densely colonized hosts. Thus it appears that these differences in behavior are
ultimately due to differences in sexual strategy. The polygynous Ips male establishes
a gallery system under an area of bark (resource defense). Newly arriving males can
avoid competition by avoiding high concentrations of pheromone and search for the
less densely colonized areas on the "weakened tree". On the other hand, females
apparently rely on the male's "judgement" concerning the likelihood of competition and
resource desirability and simply want to join the harem (the more males per area, the
easier to find one; or more simply, females continue orienting up to pheromone
gradient to find the male source).
Based on these and other data (Byers 1981b), a semiochemical description of
aggregation and colonization is presented in Fig.1.
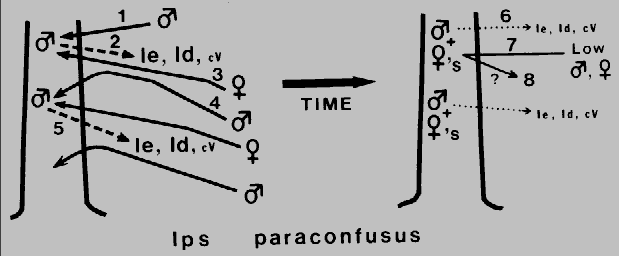
Fig. 1. Theoretical mechanism for regulation of attack density
(intraspecific competition) and termination of aggregation in Ips paraconfusus during
colonization of a ponderosa pine. The male beetle arrives first (1) and constructs a
nuptial chamber in the phloem layer. His release of ipsenol, Ie, ipsdienol, Id, and
cis-verbenol, cV (2), attracts both sexes (3), but at higher concentrations near the
source, males are inhibited in close-range orientation and thus land in adjacent areas
of lower male attack density (4). This process is repeated and serves to spread the
colonization evenly and regulate attack density (5). After males are joined by several
females, their production of the pheromone components, Ie and Id, declines rapidly
(6) and the tree becomes unattractive to beetles (7). However, it appears that an
additional mechanism (verbenone from microbes in the tree?) is needed to both
regulate density and terminate attack initiation during the later stages of colonization
(8).
However, the mechanism of
sex-specific responses to pheromone will not explain how the process of aggregation
terminates in I. paraconfusus. For instance, as the release of pheromone from the
original males declines when joined by females after a few days or so (Byers 1981b),
in theory new unmated males should continue to be attracted and then replenish the
release of pheromone, resulting in a perpetuating attack and overcrowding-but this
does not occur. It is not known what inhibits attacks later in the colonization. More
likely, however, an attack-spacing mechanism (minimum allowed distance between at-
tacks) may operate throughout the attack sequence as was indicated for I.
typographus (Byers 1984).
In the spruce bark beetle of Europe, Ips typographus, males also initiate the
attack in Norway spruce Picea abies, and are less attracted than females to
higher concentrations of their aggregation pheromone (Schlyter et al. 1987a, b). Males
and females are about equally attracted at lower release rates of the attractive
components, methylbutenol and (-)-(4S)-cis-verbenol, produced by males in the
greatest amounts during the initial phases of aggregation (Bakke et al. 1977,
Birgersson et al. 1984). (-)-a-pinene, this time from Norway spruce, is used as the
precursor to (-)-cis-verbenol (Klimetzek and Francke 1980). By comparing a range of
attractive pheromone concentrations and as well by placing passive traps at a
distance from attractive sources, it was possible to show that males were less likely
than females to reach the source, especially at the higher dosages released (Schlyter
et al. 1987a, b).
Thus, I. typographus males in Europe act in a similar way to males of I.
paraconfusus in California in regard to avoiding competition by landing in areas
adjacent to the highest pheromone release, which indicates a fully- colonized zone.
This behavioral similarity is not surprising since both species have similar polygynous
mating systems and ecological requirements. However, our understanding of the
semiochemical systems of I. typographus seems more complete than that of
the Californian counterpart. This is because males of I. typographus (unlike I.
paraconfusus) have ipsenol and ipsdienol in small amounts in their guts during the
later phases of aggregation (Vite et al. 1972, Birgersson et al. 1984) which, at least
in higher doses, inhibits attraction (Schlyter et al. 1987a). These inhibitors could signal
at close range to walking beetles that areas are occupied. Another compound,
verbenone, has been shown to inhibit attraction of both I. paraconfusus (Byers
and Wood 1980,1981) and I. typographus (Bakke 1981) and verbenone is
produced by some yeasts found in association with I. typographus (Leufven et
al. 1984). Verbenone amounts were also found to increase with time in the male
nuptial chamber walls (Leufven and Birgersson 1987). Brand et al. (1976) found that
verbenone was released by fungal metabolism in southern pine beetle galleries in
loblolly pine. Possibly verbenone indicates to I. paraconfusus, Dendroctonus
brevicomis (Byers et al. 1984), and I. typographus that a host is decadent
and has become unsuitable for colonization. Based on the above findings, a
semiochemical model of aggregation and colonization for I. typographus is
shown in Fig. 2.
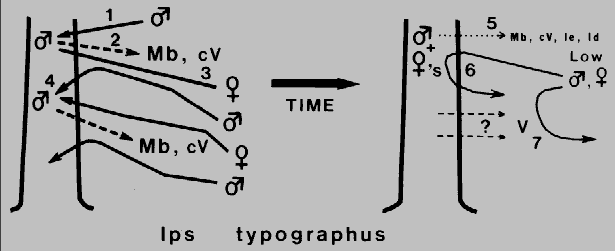
Fig. 2. Theoretical mechanism for regulation of attack density and termination of
aggregation in Ips typographus during colonization of a Norway spruce. The
male arrives first (1) and releases 2-methyl-3-buten-2-ol, Mb, and cis-verbenol, cV, (2)
which attracts both sexes from a distance (3), but as males approach high
concentrations of pheromone they become less precise than females in close-range
orientation and so land in adjacent areas (4). These sex-specific behaviors function
to spread the attack and limit attack density in a way similar to /. paraconfusus. Males
of I. typographus also reduce their release of pheromone components after
"mating" (5) but in addition they release small quantities of ipsenol, Ie, and ipsdienol,
Id, which inhibit response of both sexes probably only at close range (6). These
compounds could function to both regulate attack density and terminate aggregation
(along with a decline in attractants). However, verbenone, V, from microorganisms in
the decaying tree (7) could be the more general inhibitor responsible for termination
of aggregation.
In contrast to the Ips species, the western pine beetle of California, D. brevicomis,
does not appear to exhibit sex-specific responses to high concentrations of their
attractive components (Byers and Wood 1981, Byers et al. 1984). Exo-brevicomin,
produced by the attack- initiating females, and frontalin, produced by males, are
synergistically attractive to both sexes (Silverstein et al. 1968, Kinzer et al. 1969).
Several host monoterpenes, such as myrcene, will increase the attraction slightly
(Bedard et al. 1980). Verbenone was shown to inhibit the attraction of D. brevicomis
to its aggregation pheromone and was suggested to be produced by males later in
order to terminate aggregation (Renwick and Vite 1970). However, it was later found
that verbenone is contained in males in the largest quantities at the time of landing
and its content in the guts declines more rapidly during colonization than any of the
other components in either males or females (Byers and Wood 1980, Byers 1983c,
Byers et al. 1984). Thus, verbenone from males probably is used as a close-range
signal to indicate that the immediate area or gallery is occupied by a pair.
(-)-trans-verbenol is produced in both sexes of D. brevicomis equally from the host
monoterpene (-)-a- pinene, but in nature females typically contain significantly more
than males (Byers 1983c, Byers et al. 1984). Both sexes contain major amounts of
trans-verbenol
(comparable to peak amounts of the attractants) for the entire aggregation period and
for some time thereafter (Byers et al. 1984). Trans-verbenol was shown to inhibit only
the female's close-range orientation and entering of beetle-sized holes into a trap with
attractants. The inhibitor did not reduce the longer-range attraction at the dosages
used (Byers 1983c). Because of these results, Byers et al. (1984) hypothesized that
females utilize trans-verbenol, primarily produced by colonizing females, to avoid areas
heavily infested with conspecifics. Furthermore, while amounts of the attractants in the
hindguts of females declined to subliminal levels after a week or more of colonization,
the content of trans-verbenol was still relatively high. This would explain the
termination of aggregation. How- ever, the picture is probably more complex than this
as males produce ipsdienol from myrcene in the tree in quantities which probably have
a short-range inhibitory effect on both sexes (Byers et al. 1984). Finally, as discussed
above, verbenone release from microbial degradation of host tissue surrounding the
galleries could also indicate to host-seeking beetles that this area is unsuitable. In Fig.
3, a diagrammatic view of the olfactory mechanisms which function in reducing
competition and termination of aggregation is shown.
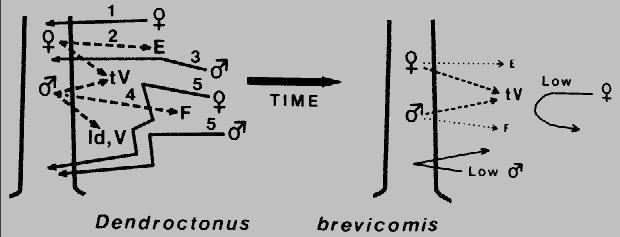
Fig. 3. Theoretical mechanism for regulation of attack density (intraspecific
competition) and termination of aggregation in Dendroctonus brevicomis during
colonization of a ponderosa pine. The female beetle arrives first (1) and bores into the
trunk and after feeding produces exo-brevicomin, E (2), which primarily attracts males
(3). Males, upon locating a female gallery, soon release frontalin, F (4), which
synergizes with E to elicit a mass aggregation (5). However, at the same time females
and males produce trans-verbenol (tV) and males pro- duce verbenone (V) and
(+)-ipsdienol (Id). At close range these compounds apparently inhibit the attraction of
beetles to E and F (t V appears to primarily affect females while Id and V affect both
sexes), which would regulate the attack density. After several days the production and
release of E and F diminishes to unattractive levels (at long range). The few fe- males
attracted during this latter period may be inhibited from attacking by the still significant,
although reduced levels of t V. The few males would not find any unpaired females
and so would continue searching elsewhere.
Olfactory mechanisms and resource partitioning for avoiding interspecific
competition Many of the inhibitory pheromones and attractive pheromone components
that are reliable indicators of higher intraspecific competition on areas of a pine tree
appear also to be useful as interspecific messages (allomones) for reducing
competition between cohabiting species. In the southern half of the Sierra of California,
D. brevicomis and I. paraconfusus are sympatric and compete for the phloem tissue
of ponderosa pine. They compete with a third beetle, I. pini, in the northern half
of the Sierra (mountains) and through the Cascade range into Oregon. In Oregon and
Idaho, D. brevicomis and I. pini are predominant. The situation is
complicated by a fourth major competitor, D. ponderosae, which in northern
California, Oregon, Idaho, and Montana competes with these species on ponderosa
or with I. pini in lodgepole pine P. contorta. There is some niche separation
between these species as the Dendroctonus species when found alone attack
the lower two-thirds or more of the tree while the Ips species when alone
usually attack the upper half or more (Miller and Keen 1960, Strubble and Hall 1955).
However, on larger logging debris, smaller wind-thrown trees, and in a broad overlap
area on standing trees there are habitats eminently suitable for all the species. In spite
of this, each species maintains a surprising degree of conspecific "purity" (often
100"/o, J. A. Byers unpubl.) in mutually colonized trees, and gradations from one
species to another along the tree trunk are described as abrupt (Miller and Keen 1960,
Birch and Wood 1975, Byers and Wood 1980).
Verbenone released by male D. brevicomis, in addition to its intraspecific
effects, appears to inhibit the response of its competitor, I. paraconfusus, to its
pheromone (Byers and Wood 1980, 1981). Verbenone was also found in I. pini
males from Idaho that had fed in red pine logs P. resinosa, but Lanier et al.
(1980) could not ascribe any "biological activity" to the compound. However, in view
of these reports, it seems that the observed inhibition of attraction of I.
paraconfusus to its pheromone by infested logs of I. pini (Birch and Wood
1975) is due in part to verbenone, a major component in I. pini (Vite et al.
1972, Lanier et al. 1980). Earlier it was believed that (-)-ipsdienol from I. pini
was solely responsible for inhibition of I. paraconfusus (Light and Birch 1979).
Thus, the behavioral effect of verbenone on I. paraconfusus may have resulted
from selection pressures to reduce interspecific competition from both D.
brevicomis and I. pini (Fig.4).
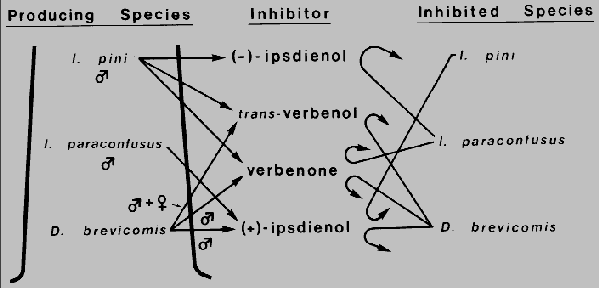
Fig. 4. Inhibition of the attraction response to conspecific pheromone by
pheromones/kairo-allomones produced by three sympatric bark beetles
Dendroctonus brevicomis, Ips paraconfusus, and I. pini which may
function to reduce interspecific competition for their host ponderosa pine in California.
Response inhibition by trans-verbenol, verbenone, and (+)- ipsdienol may also reduce
intraspecific competition in D. brevicomis.
Furthermore, the inhibitory effects of
verbenone on D. brevicomis could be the result of selection pressures from
both intraspecific competition and interspecific competition exerted by I. pini.
This could also be true of the inhibitory effects of trans-verbenol on D. brevicomis
(Fig.4) which is produced in significant amounts by D. brevicomis (Byers 1983c), by
I. pini (Vite et al. 1972, Lanier et al. 1980) and by D. ponderosae (Pitman et al. 1968).
Again, the (+)-ipsdienol produced by male I. paraconfusus (Silverstein et al.
1966) and by male D. brevicomis in the early stages of colonization may
function both to reduce intraspecific competition in both species (Byers 1982, Byers
1983b) and to reduce interspecific competition from I. pini in both species, Fig.4
(Birch et al. 1980, Byers 1982).
Therefore, the above discussion argues for olfactory systems to evolve as a result
of the relative benefits of multiple functions (intra- and interspecific). Because of
evolutionary constraints on sensory complexity and for reasons of energy efficiency,
the coevolution of various olfactory systems of these species would favor the sharing
of certain behavioral chemicals. Once a specific chemical was established and
recognized for a particular function in one species, such as avoidance of intraspecific
competition, then individuals of other species might be selected that use this
compound to avoid interspecific competition and then intraspecific competition as well.
Concurrently, there would be a selection of individuals with biosynthetic pathways that
produce the appropriate behavioral chemicals, and energy considerations should favor
a ready source of precursor, the host monoterpenes. In this mutually reinforcing type
of selection, (+)-ipsdienol may have become inhibiting to several bark beetle species
of California. Similarly, verbenone may have become inhibiting to many species spread
world-wide. However, it is increasingly apparent that verbenone may have been
produced "first" during evolution by degrading microorganisms that indicated to beetles
the presence of unsuitable host material, and then subsequently used additionally by
them as pheromones and allomones that indicated competition.
Selection of attack sites to avoid competition
A beetle should be able to make a more precise determination of the likelihood of
competition after landing, and as pointed out above, some of the olfactory mechanisms
appear to operate while walking (Byers 1983b, c). It would certainly be advantageous
for a beetle to avoid initiating an attack too close to other established attacks. An early
observation that beetles may space their attacks was made by Miller and Keen (1960)
who summarized reports of natural attack densities of D. brevicomis and found
them to vary from 0.59 to 2.32 dm` but "always within certain limits". They further
stated that "beetles seem instinctively to distribute their attacks so that overcrowding
does not occur in any particular bark area". Later, the attack patterns of D.
ponderosae, D. frontalis, D. pseudotsugae, Tomicus piniperda, and I.
typographus were analyzed and found to be more uniformly, regularly spaced than
would be expected if the patterns were random (Shepard 1965, Safranyik and
Vithayasai 1971, Mayyasi et al. 1976, Hedden and Gara 1976, Nilssen 1978, Byers
1984).
Recently, the attack patterns of I. typographus at three different densities
were compared with a computer model which randomly simulated the same densities,
but at several degrees of uniform spacing, such that the nearest neighbor distances
overlapped those of the natural patterns (Byers 1984). By this method and regression
analysis, a certain distance of spacing between attacks was found (2.5 cm) which
could explain the nearest neighbor average distances between attacks for all three
natural densities (Fig. 5).
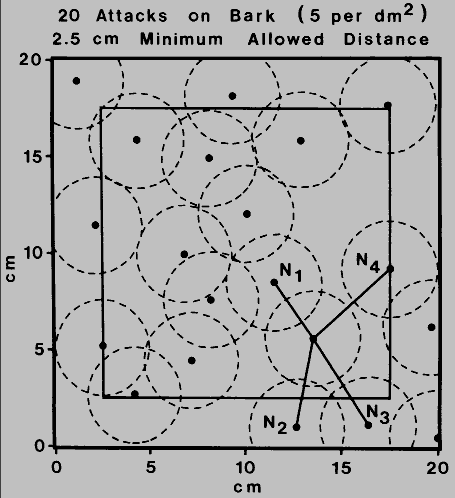
Fig. 5. Computer simulation of bark beetle attacks on bark surface (5 dm-
2). The attacks of 20 beetles in a 20 x 20 cm area are represented such that
all attacks are separated by at least 2.5 cm (the calculated minimum allowed distance
for Ips typographus, Byers 1984). Attacks occurring outside a perimeter border
of 2.5 cm (inner square) were not considered except as neighbors in determining the
average distances (cm) to the four nearest neighboring attacks (N = 11):
n1 = 3.33, n2 = 3.96, n3 = 4.74 and
n4 = 5.41. The average expected distances to the four nearest
neighboring attacks, if the distribution was random, are 2.36, 3.40, 4.43 and 5.74 for
n1, to n4 respectively (Byers 1984).
Since the estimated minimum allowed distance of 2.5 cm did
not differ appreciably at the three densities, this may indicate that it is behaviorally
controlled and inherently fixed. There would be, as expected, individual genetic
variation. Thus, the "minimum allowed distance" may be a population or species
parameter and allow comparisons with other species. In each species there may be
a specific upper density which can never be exceeded because of the particular
spacing requirements. Therefore, at an upper density a spacing mechanism would prevent new attacks from
occurring, and then termination of the aggregation would result since it has been
shown that production of pheromones declines after mating in several species
(Hughes 1973, Coster and Vite 1972, Gore et al.1977, Byers 1981b, Byers et al. 1984,
Birgersson et al. 1984).
The nature of the supposed behavioral spacing mechanism in I.
typographus, and in other beetles, is virtually unknown. One possibility is the
avoidance of higher concentrations of male pheromone attractants emanating from an
attack site, as discussed above for I. paraconfusus and I. typographus.
Another possibility is that ipsenol/ipsdienol and verbenone (Bakke 1981, Schlyter et
al. 1987a) inhibit beetles from attacking too close to others, but these compounds may
be produced too late in the attack sequence (Birgersson et al.1984) to be of much use
in spacing and avoiding competition. Stridulation by beetles has been suggested to
play a role in spacing of attacks usually by the induction of pheromone inhibitor
release (Rudinsky and Michael 1973, Rudinsky et al. 1976, Hedden and Gara 1976).
I. typographus is supposedly able to stridulate (Rudinsky 1979), but stridulatory
organs typical of other Ips (Barr 1969) are not apparent nor can stridulatory sounds
by the beetle be heard (while male D. brevicomis and T. piniperda and
female I. paraconfusus are easily heard). Other possible mechanisms are
avoidance of frass piles of beetles by olfaction or simply by visual inspection. Finally,
it may be that beetles prefer to attack bark surface structures that are uniformly
distributed, as suggested by Shepard (1965) for D. ponderosae on lodgepole
pine.
Safranyik and Vithayasai (1971) concluded that the regular attack pattern of D.
ponderosae in nature is due to the regular pattern of bark niches. They drilled holes
in a 2.5-cm hexagonal pattern in logs and found that beetles only chose the holes to
initiate galleries and that the proximity of previously established attacks did not affect
the choice of a hole. However, unoccupied drilled holes are not normally encountered
by beetles in nature and once entered may arrest the beetle and shortcut the natural
behavioral sequence (Borden 1974). Thus, any behavioral spacing mechanisms for
boring holes that is dependent on the presence of other beetles might have been
obviated under these conditions. Furthermore, in most cases bark niches seem much
more dense than the attacks of beetles. Obviously, much further work is needed to
narrow the range of possibilities.
Gallery construction and re-emergence to avoid competition
Assuming a beetle has tried to locate areas of lower attack density during its flight,
then has avoided attacking near sites of others, but still finds crowded conditions
under the bark, it has a few options left. For instance, the galleries of various bark
beetle species are commonly observed to circumscribe other galleries of their own or
other species (Schmitz and Rudinsky 1968, Wagner et al. 1981). For example, of 27
I. typographus male attacks that I inspected on Norway spruce trees in
Denmark (two examples shown in Fig.6), six female galleries "bent away from" (>30ø)
another conspecific gallery when about 3 mm distant, while eight turned similarly
before reaching nuptial chambers of P. chalcographus, and the remaining 48,
which did not encounter other galleries, were not so bent (P < 0.01, Chi-square test).
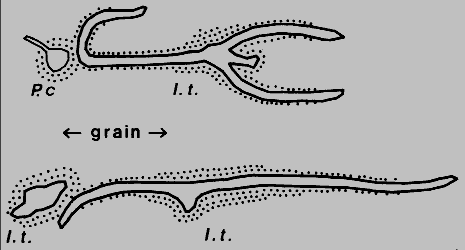
Fig. 6. Avoidance of parent galleries and nuptial chambers of conspecific or
heterospecific individuals, Ips typographus (I.t.) and Pityogenes
chalcographus (P.c.) in Norway spruce, Grib skov, northern Sjaelland, Denmark
(May 1984). Sketched from bark-stripped galleries, stipled areas represent oxidative
discoloration of phloem tissue.
The distribution of female galleries of 1, 2, 3, or 4 per male was 3, 14, 9, and 1,
respectively. It seems that chemicals in the oxidative discoloration zone surrounding
larval and adult galleries and nuptial chambers, as well as saprophytic fungi, may be
responsible for the avoidance of tunneling adults. It seems quite possible that larvae
use a similar mechanism to avoid other larval mines (which also appear to avoid each
other). The mechanism could be either gustatory or olfactory or both, but next to
nothing is known about the specifics. Schmitz and Rudinsky (1968) and DeJong and
Saarenmaa (1985) also suggest that larvae, and perhaps adults, may avoid each other
under the bark by means of vibrations (sounds) from the movement and feeding of
adjacent larvae (and adults), although no acoustical and behavioral evidence has been
found to date. Wagner et al. (1981) concluded that since the slope of the decay curve
of gallery length with increasing female D. frontalis density was more gradual
than the egg/density curve, this indicated females avoided other galleries as they
proceeded in order to lay eggs. However, these mathematical functions do not provide
direct evidence that females can disperse their galleries evenly to reduce competition.
Bark beetles will re-emerge earlier at high attack densities than at lower densities
(McMullen and Atkins 1961, Ogibin 1973, Coulson et al. 1978, Anderbrant et al. 1985).
Perhaps the adult beetle decides to re- emerge after repeatedly tunneling into areas
unsuitable for larval development (due to ageing, fermentation, and drying of host
tissue as well as deterioration from feeding/defecation). Wagner et al. (1982) provide
evidence that later attacking D. frontalis pairs were likely to stay for shorter periods
and lay fewer eggs in small bark slabs than initially attacking pairs. The effect of host
age and density could not be separated, however, and pair site positioning was not
randomized so that the last pairs always had the log-end areas. Kirkendall (1983)
formulated three reasons why bark beetles may re-emerge: (1) the bankruptcy
hypothesis, beetles can not lay more eggs unless they pause to restore energy
reserves by feeding as they re-emerge; (2) the greener pastures hypothesis, after
laying a minimal brood it is better to spread the risks by establishing a second brood
in another distant area; and (3) the overcrowding hypothesis, beetles keep laying eggs
unless the habitat becomes crowded such that new progeny would be adversely
affected. Hypothesis (1) does not seem to explain re-emergence since adults could
restore reserves without re-emerging, although it appears they could replenish energy
supplies by feeding during re-emergence. Hypotheses (2) and (3) are probably both
operating and of different relative importance based on the environmental conditions.
The decision to re-emerge, according to theory, must balance the risks of the
increasingly unfavourable conditions under the bark with the risks of a second
host-seeking flight and brood establishment. The "decision threshold" is probably
genetically variable and averaged through time by natural selection during periods of
epidemic and endemic population levels.
Interacting components regulating competition
Competition in bark beetles is affected by several ecological factors as shown in
Fig.7.
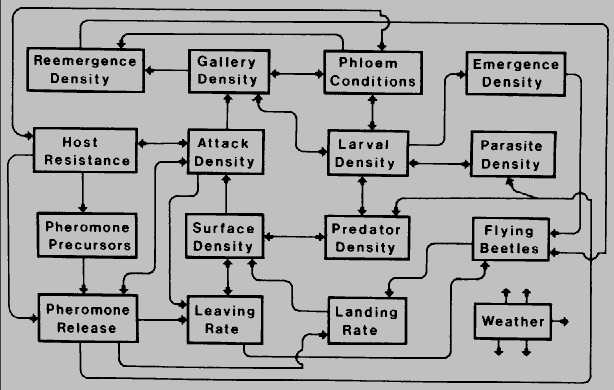
Fig. 7. Schematic presentation of theoretical interactions be- tween ecological
components which may influence the intra- and interspecific competition of bark
beetles in host trees. Weather affects all components to some extent.
Initially, host tree properties such as monoterpene quality and quantity affect the
pheromone precursor availability and the physiological processes within the beetle
such as pheromone biosynthesis and general toxicity. These influence the release of
attractive and inhibitory pheromones that affect leaving and landing rates of the beetle,
in order to avoid competition, as well as attraction of predators and parasites. The
density of bark beetles on the tree is affected by the flying population of beetles (and
leaving and landing rates) as well as predator populations. The attack density, which
is dependent on the surface density of beetles, host resistance, and pheromone
release, affects gallery density which in turn can affect re-emergence, phloem
conditions, and larval density. Larval density and emergence density, the reproductive
outcome, are primarily affected by gallery density, phloem conditions, and predators
and parasites. An understanding of these interactions, and the mechanisms affected,
will help focus our questions of how beetles avoid competition.
Practical or applied considerations
While the foregoing interactions among beetles and host will provide interesting
hypotheses and background for future basic research, are there any prospects for
applying this knowledge to protect trees? The intraspecific attractive pheromones have
been used to trap-out large numbers of D. brevicomis and I.
typographus in attempts to reduce tree mortality (Wood et al. 1985, Lie and Bakke
1981). McLean and Borden (1979) used pheromone baits to catch an estimated 65.1%
of the total population of Gnathotricus sulcatus, an ambrosia beetle, in a
suppression program at a sawmill. Other programs have used attractive baits to keep
emerging brood adults within an active infestation which other- wise would have mass
attacked trees in nearby areas (D. pseudotsugae - Pitman 1973, D. frontalis -
Richerson et al. 1980). Intraspecific inhibitory pheromones (for reducing competition)
have been used to protect trees so that beetles apparently perceive them as already
colonized or decadent and thus do not respond or are repelled. MCH
(3-methyl-2-cyclohexene-1-one) has been used in this way to protect Douglas-fir trees
from attack by D. pseudotsugae (Furniss et al.1974, St. Clair et al. 1977, Furniss et
al. 1977, Hedden and Pitman 1978) and Sitka spruce from D. rufipennis
(Rudinsky et al. 1974). Larger test plots of windthrown Douglas-fir were treated with
granular controlled-release formulations of MCH which significantly reduced D.
pseudotsugae infestation of windthrown trees and adjacent stands compared with
nearby check areas (McGregor et al. 1985, Furniss et al. 1982). They found that
beetle populations were reduced by up to 96.4 % and protection of trees was indicated
for up to two years following the MCH treatment. Endo- and exo-brevicomin have
significantly reduced landing of southern pine beetle on host pines (Payne et al. 1977).
Possibly one could combine repulsion from host trees by inhibitory pheromones or
allomones with attraction to traps by pheromones in a very effective control system.
Another way may be to attract beetles to a tree with pheromones and then confuse
or disrupt the attack spacing mechanism to create a perpetual attack in which
overcolonization destroys the adults and brood (a super trap-tree). Since we
understand little about attack-spacing mechanisms, one can not be more specific
about the methods. Furthermore, we need to determine the mechanisms by which
aggregation is terminated (Byers et al. 1984) or whether "old" trees are perceived as
unsuitable. A better understanding of the attractant and inhibitory properties of
pheromone blends will also help in the design of monitoring and population-reducing
traps. For example, the synthetic pheromone bait used to reduce populations of I.
typographus in Europe consists of a three-component blend, cis-verbenol,
methyl-butenol, and ipsdienol (Bakke et al. 1983). However, ipsdienol is not produced
in the male during colonization except after females have joined them, and then only
in trace nanogram quantities (Bakke 1976, Birgersson et al. 1984). Ipsdienol has
probably no observable behavioral effect on flying beetles at this level, and at
commercial bait levels may reduce response (Schlyter et al. 1987a). This could be
because I. duplicatus produces large amounts of ipsdienol (Bakke 1975, G. Birgersson
unpubl.) which may act interspecifically to inhibit I. typographus. Aside from the
extra cost of including ipsdienol, it also attracts the bark beetle predator
Thanasimus formicarius to traps. Thus, a binary blend (without ipsdienol) for
I. typographus may be more attractive, economical, and biologically
advantageous.
Acknowledgements - I am grateful to O. Anderbrant and F. Schlyter for
helpful advise and stimulating discussions concerning this review (to 1986). Support
for the work came from the Swedish research project "Odour Signals for Control of
Pest Insects" (NFR, FRN, SJFR, STU); and Swedish (STU 84- 3937) and American
(NSF/INT-R503520) research grants.
JOHN A. BYERS
Department of Animal Ecology, Lund University, SE-223 62 Lund, Sweden
Present address: 
|
|---|
References
Anderbrant, O., Schlyter, F. and
Birgersson, G. 1985. Intraspecific competition affecting parents and offspring in the
bark beetle Ips typographus. - Oikos 45: 89-98.
Bakke, A. 1975. Aggregation pheromone in the bark beetle, Ips duplicatus
(Sahlberg). - Norw. J. Entomol. 22: 67-69.
Bakke, A. 1976. Spruce bark beetle, Ips typographus: Pheromone production
and field response to synthetic pheromones. - Naturwissenschaften 63: 92.
Bakke, A. 1981. Inhibition of the response in Ips typographus to the
aggregation pheromone: field evaluation of verbenone and ipsenol. - Z. Angew.
Entomol. 92: 172-177.
Bakke, A., Froyen, P. and Skattebol, L. 1977. Field response to a new pheromonal
compound isolated from Ips typographus.- Naturwissenschaften 64: 98.
Bakke, A., Saether, T. and Kvamme, T. 1983. Mass trapping of the spruce bark
beetle Ips typographus. Pheromone and trap technology. - Meddelelser fra
Norsk institutt for skogforskning 38: 1-35.
Barr, B. A. 1969. Sound production in Scolytidae (Coleoptera) with emphasis on the
genus Ips. - Can. Entomol. 101: 636-672.
Beaver, R. A. 1974. Intraspecific competition among bark beetle larvae (Coleoptera:
Scolytidae). -J. Anim. Ecol. 43: 455-467.
Bedard. W. D., Wood, D. L., Tilden. P. E., Lindahl, K. Q., Jr., Silverstein, R. M. and
Rodin, J. O. 1980. Field response of the western pine beetle and one of its predators
to host- and beetle-produced compounds. - J. Chem. Ecol. 6: 625-641.
Berryman, A. A. 1974. Dynamics of bark beetle populations: towards a general
productivity model. - Environ. Entomol. 3: 579-585.
Birch, M. C. and Wood, D. L. 1975. Mutual inhibition of the attractant pheromone
response by two species of Ips. - J. Chem. Ecol. 1: 101-113.
Birch, M. C., Light, D. M., Wood, D. L., Browne, L. E., Silverstein, R. M., Bergot, B.
J., Ohloff, G., West, J. R. and Young, J. C. 1980. Pheromonal attraction and
allomonal interruption of Ips pini in California by the two enantiomers of
ipsdienol. - J. Chem. Ecol. 6: 703-717.
Birgersson, G., Schlyter, F., Löfqvist, J. and Bergström, G. 1984. Quantitative
variation of pheromone components in the spruce bark beetle Ips typographus from
different attack phases. - J. Chem. Ecol. 10: 1029-1055.
Borden, J. H. 1974. Aggregation pheromones in the Scolytidae. - In: Birch, M. C.
(ed.). Pheromones. North-Holland, Amsterdam, pp. 135-160.
Brand, J. M., Bracke, J. W., Britton, L. N., Markovetz, A. J. and Barras, S. J. 1976.
Bark beetle pheromones: Production of verbenone by a mycangial fungus of
Dendroctonus frontalis (Col., Scolytidae). - J. Chem. Ecol. 2: 195-199.
Byers, J. A. 1981a. Pheromone biosynthesis in the bark beetle Ips
paraconfusus, during feeding or exposure to vapours of host plant precursors.
- Insect Biochem. I1: 563-569.
Byers, J. A. 1981b. Effect of mating on terminating aggregation during host
colonization in the bark beetle, Ips paraconfusus. - J. Chem. Ecol. 7:
1135-1147. Byers, J. A. 1982. Male-specific conversion of the host plant compound,
myrcene, to the pheromone, (+)-ipsdienol, in the bark beetle, Dendroctonus
brevicomis. - J. Chem. Ecol. 8: 363- 371.
Byers, J. A. 1983a. Influence of sex, maturity and host substances on pheromones
in the guts of the bark beetles, Ips paraconfusus and Dendroctonus
brevicomis. - J. Insect Physiol. 29: 5-13.
Byers, J. A. 1983b. Sex-specific responses to aggregation pheromone: Regulation
of colonization density by the bark beetle Ips paraconfusus. - J. Chem. Ecol.
9: 129-142.
Byers, J. A. 1983c. Bark beetle conversion of a plant compound to a sex-specific
inhibitor of pheromone attraction. - Science 220: 624-626.
Byers, J. A. 1984. Nearest neighbor analysis and simulation of distribution patterns
indicate an attack spacing mechanism in the bark beetle, Ips typographus
(Coleoptera: Scolytidae). - Environ. Entomol. 13: 1191-1200.
Byers, J. A. and Wood, D. L. 1980. Interspecific inhibition of the response of the bark
beetles, Dendroctonus brevicomis and Ips paraconfusus. - J. Chem.
Ecol. 6: 149-164.
Byers, J. A. and Wood, D. L. 1981. Interspecific effects of pheromones on the
attraction of the bark beetles, Dendroctonus brevicomis and Ips
paraconfusus in the laboratory. - J. Chem. Ecol. 7: 9-18.
Byers, J. A., Wood, D. L., Browne, L. E., Fish, R. H., Piatek, B. and Hendry, L. B.
1979. Relationship between a host plant compound, myrcene and pheromone
production in the bark beetle Ips paraconfusus. - J. Insect Physiol. 25: 477- 482.
Byers, J. A., Wood, D. L., Craig, J. and Hendry, L. B.1984. Attractive and inhibitory
pheromones produced in the bark beetle. Dendroctonus brevicomis, during host
colonization: Regulation of inter- and intraspecific competition. - J. Chem. Ecol. 10:
861-877.
Byers, J. A., Birgersson, G., Löfqvist, L. and Bergström, G. 1988. Synergistic
pheromones and monoterpenes enable aggregation and host recognition by a bark
beetle. - Naturwissenschaften 75: 153-155.
Coster, J. E. and Vite, J. P. 1972. Effects of feeding and mating on pheromone
release in the Southern pine beetle. - Ann. Entomol. Soc. Amer. 65: 263-266.
Coulson, R. N., Fargo, W. C., Pulley, P. E., Foltz, J. L., Pope, D. N., Richerson, J.
V. and Payne, T. L. 1978. Evaluation of the re-emergence process of parent adult
Dendroctonus frontalis (Coleoptera: Scolytidae). - Can. Entomol. 110: 475-
486.
DeJong, M. C. M. and Saarenmaa, H. 1985. A mechanistic simulation model for the
movement of hark beetle larvae (Coleoptera, Scolytidae). - Ecol. Model. 27: 109-138.
Eidmann, H. H. and Nuorteva, M. 1968. Der Einfluss der Siedlungsdichte und
anderer Faktoren auf die Anzahl Nachkommen von Blastophagus piniperda
L. (Coleoptera: Scolytidae). - Ann. Entomol. Fenn. 34: 135-148.
Furniss, M. M., Daterman, G. E., Kline, L. N., McGregor, M. D., Trostle, G. C.,
Pettinger, L. F. and Rudinsky, J. A. 1974. Effectiveness of the Douglas-fir beetle
antiaggregative pheromone methylcyclohexenone at three concentrations and spacings
around felled host trees. - Can. Entomol. 106: 381-392.
Furniss, M. M., Young, J. W., McGregor, M. D., Livingston, R. L. and Hamel, D. R.
1977. Effectiveness of controlled-release formulations of MCH for preventing
Douglas-fir beetle (Coleoptera: Scolytidae) infestations in felled trees. -Can. Entomol.
109: 1063-1069.
Furniss, M. M., Markin, G. P. and Hager, V. J.1982. Aerial application of Douglas-fir
beetle antiaggregative pheromone: Equipment and evaluation. - Gen. Tech. Rep.
INT-137. Ogden, UT: USDA, Intermountain Forest and Range Exp. Station.
Gore. W. E., Pearce, G. T., Lanier, G. N., Simeone, J. B., Silverstein, R. M.,
Peacock, J. W. and Cuthbert, R. A. 1977. Aggregation attractant of the European
elm bark beetle, Scolytus multistriatus: Production of individual components
and related aggregation behavior. - J. Chem. Ecol. 3: 429-446.
Hedden, R. L. and Gara, G.1. 1976. Spatial attack pattern of a Western Washington
Douglas-fir beetle population. - Forest Sci. 22: 100-102.
Hedden, R. L. and Pitman, G. B. 1978. Attack density regulation: A new approach
to the use of pheromones in Douglas-fir beetle population management. - J. Econ.
Entomol. 71: 633-637.
Hendry, L. B., Piatek, B., Browne, L. E., Wood, D. L., Byers, J. A.. Fish, R. H. and
Hicks. R. A. 1980. In vivo conversion of a labelled host plant chemical to
pheromone of the bark beetle Ips paraconfusus. - Nature (Lond.) 284: 485-
487.
Hodges, J. D., Elam, W. W., Watson, W. F. and Nebeker, T. E. 1979. Oleoresin
characteristics and susceptibility of four southern pines to southern pine beetle
(Coleoptera: Scolytidae) attacks. - Can. Entomol. 111: 889-896.
Hughes, P. R. 1973. Dendroctonus: Production of pheromones and related
compounds in response to host monoterpenes. - Z. Angew. Entomol. 73: 294-312.
Hughes, P. R. 1974. Myrcene: A precursor of pheromones in Ips beetles. -
J. Insect Physiol. 20: 1271-1275.
Kinzer, G. W., Fentiman, A. G., Jr., Page, T. F., Jr., Foltz, R. I.. Vite, J. P. and
Pitman. G. B. 1969. Bark beetle attractants: Identification synthesis and field bioassay
of a new compound isolated from Dendroctonus. - Nature (Lond.) 211:
477-478.
Kirkendall, L. R. 1983. The evolution of mating systems in bark and ambrosia
beetles (Coleoptera: Scolytidae and Platypodidae). - Zool. J. Linn. Soc. 77: 293-352.
Klimetzek. D. and Francke, W. 1980. Relationship between the enantiomeric
composition of à-pinene in host trees and the production of verbenols in Ips
species. - Experientia 36: 1343-1345.
Lanier, G. N., Claesson, A., Stewart. T., Piston. J. J. and Silverstein, R. M. 1980.
Ips pini: The basis for interpopulation differences in pheromone biology. - J.
Chem. Ecol. 6: 677-687.
Leufven. A. and Birgersson, G. 1987. Quantitative variation of different
monoterpenes around galleries of Ips typographus (Coleoptera: Scolytidae)
attacking Norway spruce. - Can. J. Bot. 65: 1038-1044.
Leufvén, A., Bergström, G. and Falsen, E. 1984. Interconversion of verbenols and
verbenone by identified yeasts isolated from the spruce bark beetle Ips
typographus. - J. Chem. Ecol. 10: 1349-1361.
Lie, R. and Bakke, A. 1981. Practical results from the mass trapping of Ips
typographus in Scandinavia. - In: Mitchell, E. R. (ed.). Management of insect
pests with semiochemicals, Plenum Press, New York, pp. 175-181.
Light, D. M. and Birch, M. C. 1979. Inhibition of the attractive pheromone response
in Ips paraconfusus' by (R)-(-)-ipsdienol. - Naturwissenschaften 66: 159.
Light, D. M., Birch, M. C. and Paine, T. D. 1983. Laboratory study of intraspecific
and interspecific competition within and between two sympatric bark beetle species,
Ips pini and I. paraconfusus. - Z. Angew. Entomol. 96: 233-241.
Mayyasi, A. M., Coulson, R. N., Foltz, J. L., Hain, F. P. and Martin, W. C. 1976.
Functional description of within-tree larval and progeny adult populations of
Dendroctonus frontalis (Coleoptera: Scolytidae). - Can. Entomol. 108:
363-372.
McGregor, M. D., Oakes, R. D. and Meyer, H. E. 1985. Evaluation of Douglas-fir
mortality from Douglas-fir beetle from 1982 through 1984 following MCH application.
- USDA For. Serv. Missoula, MT, Rep. 85-7.
McLean, J. A. and Borden, J. H. 1979. An operational pheromone-based suppression
program for an ambrosia beetle, Gnathotrichis sulcatus, in a commercial
sawmill. - J. Econ. Entomol. 72: 165-172. McMullen, L. H. and Atkins, M. D. 1961.
Intraspecific competition as a factor in the natural control of the Douglas-fir beetle.
- Forest Sci. 7: 197-203.
Miller, J. M. and Keen, F. P. 1960. Biology and control of the western pine beetle.
- U.S. Dep. Agric. Misc. Publ. No. 800.
Nilssen, A. C. 1978. Spatial attack pattern of the bark beetle Tomicus
piniperda L. (Col., Scolytidae). - Norw. J. Entomol. 25: 171-175.
Ogibin, B. N. 1973. Effect of population density on fertility in Ips typographus
L. - Translated from Ekologiya No. 5, pp. 66-72.
Paine, T. D., Birch, M. C. and Svihra, P. 1981. Niche breadth and resource
partitioning by four sympatric species of bark beetles (Coleoptera: Scolytidae). -
Oecologia (Berl.) 48: 1-6.
Payne, T. L., Coster. J. E. and Johnson, P. C. 1977. Effects of slow-release
formulation of synthetic endo- and exo-brevicomin on southern pine beetle flight and
landing behavior. - J. Chem. Ecol. 3: 133-141.
Pitman, G. B. 1973. Further observations on Douglure in a Dendroctonus
pseudotsugae management system. - Environ. Entomol. 2: 109-112.
Pitman, G. B., Vite, J. P., Kinzer, G. W. and Fentiman, A. F., Jr. 1968. Bark beetle
attractants: trans-Verbenol from Dendroctonus. - Nature (Lond.) 218: 168-169.
Raffa, K. F. and Berryman, A. A. 1983. The role of host plant resistance in the
colonization behavior and ecology of bark beetles (Coleoptera: Scolytidae). - Ecol.
Monogr. 53: 27-50.
Renwick, J. A. A. and Vite, J. P. 1970. Systems of chemical communication in
Dendroctonus. - Contrib. Boyce Thompson Inst. 24: 283-292.
Renwick, J. A., Hughes, P. R. and Krull, I. S. 1976. Selective production of cis- and
trans-verbenol from (-) and (+) alpha-pinene by a bark beetle. - Science 191:
199-201.
Richerson. J. V., McCarty, F. A. and Payne, T. L. 1980. Disruption of southern pine
beetle infestations with frontalure. - Environ. Entomol. 9: 90-93.
Rosenthal. G. A. and Janzen, D. H. (eds). 1979. Herbivores: Their interaction with
secondary plant metabolites. - Academic Press, New York.
Rudinsky, J. A. 1979. Chemoacoustically induced behavior of Ips
typographus (Col.: Scolytide). - Z. Angew., Entomol. 88: 537-541.
Rudinsky, J. A. and Michael, R. R. 1973. Sound production in Scolytidae: stridulation
by female Dendroctonus beetles. - J. Insect Physiol. 19: 689-705.
Rudinsky, J. A., Sartwell, C., Jr., Graves, T. M. and Morgan, M. E. 1974. Granular
formulation of methylcyclohexenone: an antiaggregative pheromone of the Douglas fir
and spruce bark beetles (Col., Scolytidae). - Z. Angew. Entomol. 75: 254- 263.
Rudinsky, J. A., Ryker, L. C., Michael, R. R., Libbey, L. M. and Morgan, M. E. 1976.
Sound production in Scolytidae: female sonic stimulus of male pheromone release
in two Dendroctonus beetles. - J. Insect Physiol. 22: 1675-1681.
Safranyik, L. and Vithayasai, C. 1971. Some characteristics of the spatial
arrangement of attacks by the mountain pine beetle, Dendroctonus
ponderosae (Coleoptera: Scolytidae), on lodgepole pine. - Can. Entomol. 103:
1607-1625.
Schlyter, F., Byers, J. A. and Löfqvist, J. 1978a. Attraction to pheromone sources
of different quantity, quality, and spacing: Density-regulation mechanisms in bark
beetle, Ips typographus. - J. Chem. Ecol. 13: 1503-1523.
Schlyter, F., Löfqvist, J. and Byers, J. A. 1987b. Behavioural sequence in the
attraction of the bark beetle Ips typographus to pheromone sources. - Physiol.
Entomol. 12: 185-196.
Schmitz, R. F. and Rudinsky, J. A. 1968. Effect of competition on survival in
Western Oregon of the Douglas-fir beetle. - Res. Pap. 8, Forestry Research
Laboratory, School of Forestry, Oregon State Univ., Corvallis, OR.
Shepard, R. F. 1965. Distribution of attacks by Dendroctonus ponderosae
Hopk. on Pinus contorta Dougl. var. latifolia Englm. - Can. Entomol.
97: 207-215.
Silverstein, R. M., Rodin, J. O. and Wood, D. L. 1966. Sex attractants in frass
produced by male Ips confusus in ponderosa pine. - Science 154: 509-510.
Silverstein, R. M., Brownlee, R. G., Bellas, R. E., Wood, D. L. and Browne, L. E.
1968. Brevicomin: Principal sex attractant in the frass of the female Western pine
beetle. - Science 159: 889-891.
Smith, R. H. 1965. Effect of monoterpene vapors on the western pine beetle. - J.
Econ. Entomol. 58: 509-510.
St. Clair, A., Goulding, R. L. and Rudinsky, J. A. 1977. Controlled-release dispensing
system for 3,2-MCH, antiaggregative pheromone of the Douglas-fir beetle (Col.:
Scolyt.). - Z. Angew. Entomol. 83: 297-300.
Strubble, G. R. and Hall, R. C. 1955. The California five-spined engraver, its biology
and control. - USDA Circular No. 964.
Sturgeon, K. B. 1979. Monoterpene variation in ponderosa pine xylem resin related
to western pine beetle predation.- Evolution 33: 803-814.
Svihra, P.1972. Population dynamics of Ips typographus in the upper Hronie
region. - Ved. Pr. Vyzh. Ustavu Lesn. Hos- pod. 18: 227-258.
Vite, J. P., Bakke, A. and Renwick, J. A. A. 1972. Pheromones in Ips (Coleoptera:
Scolytidae): Occurrence and production. - Can. Entomol. 104:1967-1975.
Wagner, T. L., Feldman, R. M., Gagne, J. A., Cover, J. D., Coulson, R. N. and
Schoolfield, R. M.1981. Factors affecting gallery construction, oviposition, and
reemergence of Dendroctonus frontalis in the laboratory. - Ann. Entomol.
Soc. Am. 74: 255-273.
Wagner, T. L., Fargo, W. S., Keeley, L. L., Coulson, R. N. and Cover, J. D. 1982.
Effects of sequential attack on gallery construction, oviposition, and re-emergence by
Dendroctonus frontalis (Coleoptera: Scolytidae). - Can. Entomol. 114: 491-502.
Wood, D. L. 1982. The role of pheromones, kairomones, and allomones in the host
selection and colonization behavior of bark beetles. - Annu. Rev. Entomol. 27:
411-446.
Wood, D. L., Browne, L. E., Bedard, W. D., Tilden, P. E., Silverstein, R. M. and
Rodin, J. O. 1968. Response of Ips confusus to synthetic sex pheromones
in nature. - Science 159: 1373- 1374.
Wood, D. L., Stark, R. W., Waters, W. E., Bedard, W. D. and Cobb, F. W., Jr. 1985.
Treatment tactics and strategies. - In: Waters, W. E., Stark, R. W. and Wood, D. L.
(eds). Integrated pest management in pine-bark beetle ecosystems. John Wiley &
Sons, New York, pp. 121-139.

 Bark beetles such as Ips typographus
at the top of the Norway spruce log, Picea abies, must compete for the relatively thin layer of bark-phloem
as seen in the brown area around the log.
Bark beetles such as Ips typographus
at the top of the Norway spruce log, Picea abies, must compete for the relatively thin layer of bark-phloem
as seen in the brown area around the log.







