Byers, J.A. Chemical Ecology of Bark Beetles. Experientia 45:271-283.
Continued...
Attack density regulation and termination of aggregation/attack
mediated by semiochemicals. In response to the pheromone from the
pioneer beetles resident in the tree, flying beetles orient to the tree, some joining
established galleries while others begin excavations in the bark. The release of
pheromone from these many new attacks increases perhaps as a logistic function
such that the resinous defenses of the tree are
overwhelmed45,68,
108,152. Once
the tree has been rendered effectively defenseless, the functional cooperation
among beetles ends and competition for the two-dimensional phloem becomes
progressively more severe as the aggregation and attack
continues2,14,34
.
This intense competition has provided strong selection pressures for the evolution
of individuals adept at avoiding or reducing the degree of competitive interactions
with conspecifics and with individuals of competing
species34,38. As mentioned
previously, I. paraconfusus males can determine the likelihood of competition
based on the strength of the pheromone signal. They avoid orientating to the
highest release sources while females prefer these areas of the
tree33. At the peak of the female landing period on a felled tree,
the male landing rate actually declined indicating that males may have been
attracted (as shown in long-range orientation through a grid of traps) but did not
land and then chose to fly elsewhere33. These behaviors would
serve to spread the colonization area in
Ips33,120,
121 and P.
chalcographus44 as well as by a more simple "spill-over"
or imprecision in orientation to pheromone. This latter mechanism may also account
primarily for the switch of attack focus to surrounding trees by D. frontalis
and other pest
species3,52,1
19.
Bark beetles appear to have behavioral mechanisms which space the attacks and
gallery systems in order to reduce competition to tolerable levels. The mechanisms
by which beetles choose whether to attack near previously attacked sites and at
what distance are poorly known. Spacing of attacks has been shown in D.
ponderosae124,11
8, D. frontalis88, D.
pseudotsugae67, T. piniperda96 and
I. typographus34. A computer method of comparing
simulated attack densities at various minimal spacings between nearest neighbors
to the natural attack patterns has been developed34. It showed
that I. typographus attacks would have the observed average nearest
neighbor distances if the beetles initiated entrance holes at least a minimum allowed
distance (MAD) of 2.5 cm from other holes. This distance was apparently the same
regardless of the natural attack density and thus appears to be an inherent
biological distance characteristic of the species34.
The possible mechanisms that account for these spaced attack distributions and
the MAD are (1) olfactory communication34, (2)
stridulation/acoustic
communication67,112
-116, (3) selection of bark
structures57,118, and
(4) visual inspection of attack sites or beetles, or various combinations of these.
Olfactory communication between Ips males has been discussed above and could
also function even more strongly at close-range. In D. pseudotsugae, an
arriving male stridulates at the female's entrance hole and stimulates her to release
MCH113,115.
The male also releases
MCH83,106 which has
an inhibitory effect at long-range and apparently also at close-
range112-115.
Verbenone, trans-verbenol and (+)-ipsdienol have been shown to inhibit
attraction of D. brevicomis to pheromone components at long-
range13,30 and (-)-trans-
verbenol at close-range32. Female stridulation alone has
been implicated in the spacing of D.
brevicomis113. Bark structure does influence the
selection of attack
site57,118 as well as
the density of attacks, but it may have little affect on the spacing patterns or
MAD.
Termination of aggregation and attack may be the result of saturation of the
available bark areas as constrained by the spacing mechanism
(MAD)34. After the sexes pair, the production of pheromone
declines as shown experimentally in I. paraconfusus28
and by observations of gut volatile contents in tree-colonizing I.
typographus17 and D.
brevicomis45. Thus, a spacing mechanism and cessation
of attractant release after mating could explain the mechanism of termination of the
aggregation. However, in T. piniperda termination may also be influenced by
verbenone emanating from infested logs with only a small portion from the beetles
themselves43,80. Similar studies
with I. typographus have shown that verbenone from microbial activity in
infested trees may play a major role in termination of the
aggregation6,15,8
1,82,119
.
In Fig. 5, semiochemical mechanisms which mediate the aggregation, attack
density and termination of aggregation are presented for four species of bark
beetle.
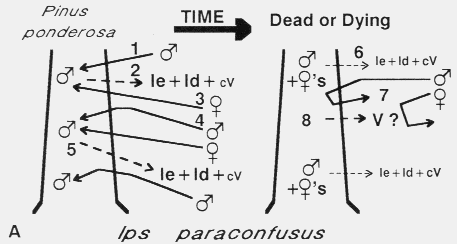
Fig. 5. Theoretical mechanisms for regulation of aggregation and attack density
(intraspecific competition) and termination of aggregation in four species of bark
beetles. A. Ips paraconfusus colonization of ponderosa pine. The male beetle
arrives first (1) and constructs a nuptial chamber in the phloem layer. His release
of ipsenol (Ie), ipsdienol (Id) and cis-verbenol (cV) [2], attracts both sexes
[3]126-128,150, but at higher concentrations near the source, males are
inhibited in close-range orientation and thus land in adjacent areas of lower male
attack density [4](Byers 1983)33. This process is repeated and serves to
evenly spread the colonization and regulate attack density [5](Byers
1983)33. After males are joined by several females, their production of the
pheromone components, Ie and Id, declines rapidly [6](Byers 1981)28 and
the tree becomes unattractive to beetles [7]. However, it appears that an additional
mechanism (verbenone, V, from microbes in the tree?) is needed to both regulate
density and terminate attack initiation during the later stages of colonization [8]. V
has been shown to inhibit attraction to natural pheromone(Byers and Wood
1980)39.
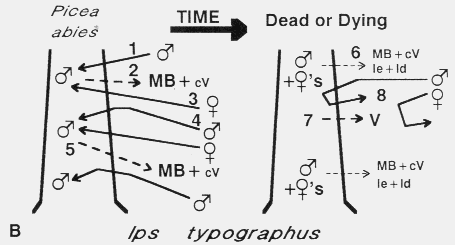
B. Ips typographus colonization of Norway spruce. The male arrives first [1] and
releases 2-methyl-3-buten-2-ol (MB) and cis-verbenol (cV) [2] which attracts
both sexes from a distance [3]8, but as males approach high
concentrations of pheromone they become less precise than females in close-range
orientation and so land in adjacent areas [4]120,121. These sex-specific
behaviors function to spread the attack and limit attack density in a way similar to
I. paraconfusus. Males of I. typographus also reduce their release of
pheromone components after "mating" [5]17 but in addition they release
small quantities of ipsenol (Ie) and ipsdienol (Id)15,17, which inhibit
response of both sexes probably only at close range [6]6,119. These
compounds could function to both regulate attack density and terminate aggregation
(along with a decline in attractants). However, verbenone (V) from microorganisms
in the bark beetle galleries [7] could be the more general inhibitor responsible for
termination of aggregation [8]6,81,82,119.
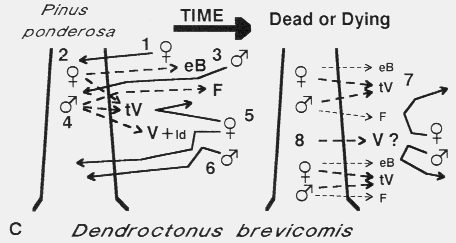
C. Dendroctonus brevicomis colonization of ponderosa pine. The female
beetle arrives first [1] and bores into the trunk and after feeding produces
exo-brevicomin (eB) [2]129, which primarily attracts males
[3]35,37. Males, upon locating a female gallery, soon release frontalin (F)
[4]74, which synergizes with eB to elicit a mass aggregation
[5]11,12. However, at the same time females and males produce trans-
verbenol (tV)32 and males produce verbenone (V) and (+)-ipsdienol
(Id)30,45. At close range these compounds apparently inhibit the attraction
of beetles to eB and F (tV appears to primarily affect females while Id and V affect
both sexes, which would regulate the attack density13,30,32. After several
days the production and release of eB and F diminishes to levels that are
unattractive at long range45. The few females attracted during this latter
period may be inhibited from attacking by the still significant, although reduced
levels of tV [7]45. The few males would not find any unpaired females and
so would continue searching elsewhere. Verbenone may also increase in release
rate as the tree decays and so inhibit beetles [8], although measurements have not
been done.
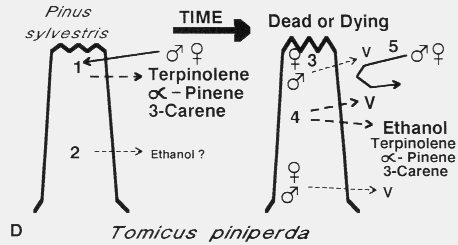
D. Tomicus piniperda colonization of Scots pine. Both sexes arrive
simultaneously in mass on the first April day above 13o C in response to
host monoterpenes, terpinolene, (± )-alpha-pinene and (+)-3-carene, released
from resinous wounds incurred during winter storms [1]47,80. Females and
males pair up under bark flakes and the females tunnel into the bark followed
immediately behind by the male. Trees that have fallen earlier in the winter and are
beginning to decay probably release ethanol which in relatively small amounts may
enhance the attraction to monoterpenes [2]76 but at higher amounts is
inhibitory76. Both sexes contain verbenone (V) in the largest amounts
shortly after entering the tree and amounts decline thereafter [3]80 while
V released from the tree continues to increase [4]43 possibly due to
microbial activity in the tissues surrounding the galleries. Ethanol may increase in
release rate due to fermentative processes (unproven) while monoterpene release
gradually decreases43. V released in traps at rates comparable to rates
released from infested sections of a tree was able to inhibit attraction of both sexes
to the attractive monoterpenes also released at natural rates43. V may
serve both to space attacks (when released from individuals) and to terminate the
aggregation (when released generally by microbial decay).
Interspecific interactions mediated by semiochemicals.
Semiochemicals function in interactions between bark beetles in primarily two ways:
(1) as allomones for interspecific communication of resource use in order to avoid
competition and (2) as kairomones for use in locating weakened hosts colonized by
another species. In California and other regions of the western United States, four
pest bark beetles compete more or less for ponderosa pine (Fig. 6).
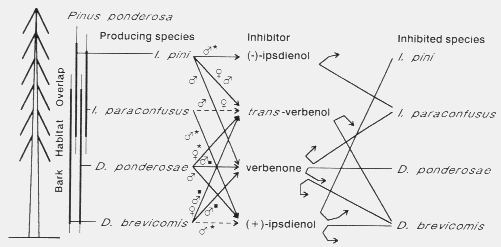
Fig. 6. Inhibition of the attraction response to conspecific pheromone by allomones
produced by four sympatric bark beetles, Dendroctonus brevicomis, D.
ponderosae, Ips paraconfusus, and I. pini which appear to
function in reducing interspecific competition for ponderosa pine in California.
Response inhibition by the pheromones trans-verbenol, verbenone, and (+)-
ipsdienol may also reduce intraspecific competition in D. brevicomis. The
asterisks indicate pheromone attractants and black squares indicate pheromone
inhibitors of attraction.
Verbenone released by male D. brevicomis, in addition to its intraspecific
effects, appears to inhibit the response of its competitor, I. paraconfusus, to
pheromone39. Verbenone has also been found in I. pini
males from Idaho that had fed in red pine logs, but no behavioral role was
ascribed79. However, I suggest here that the observed inhibition
of attraction of I. paraconfusus to its pheromone by logs infested with I.
pini19 appears due in part to verbenone, a major volatile
component in I.
pini79,142. Earlier
it was reported that only (-)-ipsdienol from I. pini was responsible for
inhibition of I. paraconfusus84. Thus, the inhibitory effect
of verbenone on I. paraconfusus may have resulted from selection pressures
to reduce interspecific competition from both D. brevicomis and I. pini
(Fig. 6). Similarly, the inhibitory effects of verbenone on D. brevicomis could
be the result of selection pressures from both intra- and interspecific competition
(from I. pini). Multiple selection pressures could also account for the inhibitory
effects of trans-verbenol on D. brevicomis32 (Fig.
6) which is produced in significant amounts by D.
brevicomis32, by I.
pini79,142 and by
D. ponderosae107. The (+)-ipsdienol produced
by male I. paraconfusus126 and by male D.
brevicomis in the early stages of colonization45 may
function both to reduce intraspecific competition in both
species30,33 and to reduce
interspecific competition from I. pini in both species (Fig.
6)20,30.
A similar situation of competitive interactions exists for four sympatric bark beetles
of southern pines (Fig. 7).
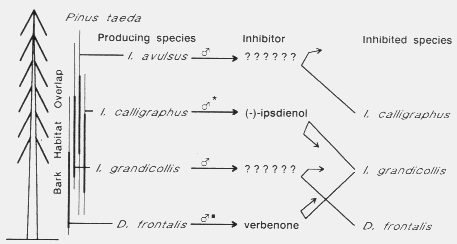
Fig. 7. Inhibition of the attraction response to conspecific pheromone by allomones
produced by four sympatric bark beetles, Dendroctonus frontalis, Ips grandicollis,
I. calligraphus, and I. avulsus which appear to function in reducing
interspecific competition for southern pines in the southeastern United
States21,136. Verbenone also acts as a pheromone in D. frontalis that
reduces response to aggregation pheromone. The asterisks indicate pheromone
attractant and the black square a pheromone inhibitor.
I. calligraphus produces (-)-ipsdienol as one of the attractive
components142,14
4 and this also acts as an allomone to inhibit I. grandicollis attraction
to its
pheromone21,143.
Allomonal volatiles from I. avulsus are inhibitory to I.
calligraphus21. Verbenone, produced by D.
frontalis106,10
9 acts as a pheromone that inhibits attraction as well as an allomone that
inhibits attraction of I. grandicollis21. The use of
allomones appears to reduce competitive interactions among the southern pine
beetles just as they do in the western pine beetles.
The bark beetles attacking pines in the southern United States are different from
their western
counterparts18,19,
39, however, in that they are cross-attractive to each others
pheromones (Fig.
8)21,136.
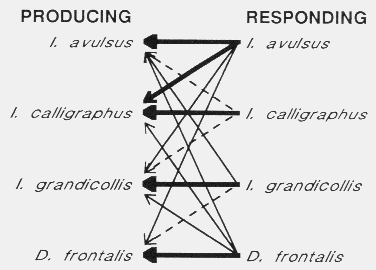
Fig. 8. Cross attraction of four sympatric bark beetles colonizing pines in the
southeastern United States21,136. Thick lined arrows indicate strong
attraction (65%-100% of conspecific attraction), thin lines indicate weaker attraction
(5-12%) and dashed lines little attraction (2-5%).
The reason for the strongest interspecific attraction, I. avulsus attraction to
pheromone of I. calligraphus, is probably that I. avulsus locates
weakened hosts already infested by I. calligraphus and that the potential
competition is tolerable since both species utilize somewhat different levels of the
tree (Fig. 7)98,136.
Therefore, the above discussion indicates that olfactory systems evolve as a
result of the relative benefits of multiple factors including avoidance of intra- and
interspecific competition. Coevolution would favor the sharing of semiochemicals so
that specific compounds could serve in communication both intra- and
interspecifically. There would also be a selection pressure for use of biosynthetic
pathways that produce the semiochemicals from host precursors, as long as the
precursors were consistently available. Thus (+)-ipsdienol, from myrcene, and
trans-verbenol (and possibly verbenone), from alpha-pinene, may have
become pheromone components and allomones for a growing list of species.
However, another hypothesis is that verbenone was already consistently present in
decaying hosts due to microbial
activity25,26,4
3,82, and then was subsequently used
over evolutionary time by bark beetles as pheromones and
allomones38.
The predators, parasites and other associated insects interacting with bark
beetles comprise a complex guild of species. During the course of aggregation of
D. brevicomis on ponderosa pine, over 100 species of insects were
collected134. Associated insects of bark beetles
locate the breeding habitat either by landing at random, or using kairomones from
the beetle (often their
pheromones)7,12,1
40,146,1
47 or other kairomones from the dying
tree76 or possibly other associated insects. The primary
requirements of a kairomone are that it be a reliable indicator of host condition or
presence and that the host (or fermentative process) can not dispense with its use.
Thus, pheromone components of bark beetles are the most likely volatiles to be
utilized as kairomones by predators and parasitoids since the adaptive advantages
for the emitter outweigh the disadvantages as a kairomone, and so no net selection
pressure for changing to other compounds will occur.
The chemical ecology of adult and larval interactions under the bark (e.g. they
appear to avoid intersecting galleries38), callow adult
movements, emergence, and overwintering (Fig. 1, d-h) are practically unknown.
Gregoire et al.66 indicated that larval D. micans maintained a
feeding aggregation in response to cis-verbenol. Fungal interactions with
bark beetles in regard to host tree
resistance68,108,
125, nutrition of larvae10
and host
unsuitability25,26,
43,81 are not well understood.
Sexual recognition and species recognition are due in part to stridulation9
but probably more so by specific cuticular
chemicals105 - a subject not yet investigated in bark
beetles.
The field of chemical ecology of bark beetles encompasses much more than can
be covered here, but it is hoped that one can appreciate that bark beetles and their
associates are among the best of model systems for the study of chemical
ecology.
1 Alcock, J., Natural selection and communication among bark beetles. Fla.
Entomol. 65 (1981) 17-32.
2 Anderbrant, O., Schlyter, F., and Birgersson, G., Intraspecific competition affecting
parents and offspring in the bark beetle Ips typographus. Oikos 45 (1985)
89-98.
3 Anderbrant, O., Schlyter, F., and Löfqvist, J., Dynamics of tree attack in the bark
beetle Ips typographus under semi-epidemic conditions, in: Integrated Control
of Scolytid Bark Beetles. in press. Eds T.L. Payne and H. Saarenmaa, Virginia
Tech. Press, Blacksburg 1989.
4 Atkins, M.D., Lipid loss with flight in the Douglas-fir beetle. Can. Entomol. 101
(1969) 164-165.
5 Baker, T.C. Pheromone-modulated movements of flying moths, in: Mechanisms
in Insect Olfaction, pp. 39-47. Eds T.L. Payne, M.C. Birch and C.E.J. Kennedy.
Clarendon Press, Oxford 1986.
6 Bakke, A., Inhibition of the response in Ips typographus to the aggregation
pheromone; field evaluation of verbenone and ipsenol. Z. Angew. Entomol. 92
(1981) 172-177.
7 Bakke, A., and Kvamme, T., Kairomone response in Thanasimus predators to
pheromone components of Ips typographus. J. Chem. Ecol. 7 (1981)
305-312.
8 Bakke, A., Fríyen, P., and Skattebíl, L., Field response to a new pheromonal
compound isolated from Ips typographus. Naturwissenschaften 64 (1977)
98.
9 Barr, B.A., Sound production in Scolytidae (Coleoptera) with emphasis on the
genus Ips. Can. Entomol. 101 (1969) 636-672.
10 Barras, S.J., Reduction of progeny and development in the southern pine beetle
following removal of symbiotic fungi. Can. Entomol. 105 (1973) 1295-1299.
11 Bedard, W. D., Silverstein, R. M., and Wood, D. L., Bark beetle pheromones.
Science 167 (1970) 1638-1639.
12 Bedard, W.D., Tilden, P.E., Wood, D.L., Silverstein, R.M., Brownlee, R.G., and
Rodin, J.O., Western pine beetle: Field response to its sex pheromone and a
synergistic host terpene, myrcene. Science 164 (1969) 1284-1285.
13 Bedard, W.D., Tilden, P.E., Lindahl, K.Q.Jr., Wood, D.L., and Rauch, P.A.,
Effects of verbenone and trans-verbenol on the response of Dendroctonus
brevicomis to natural and synthetic attractant in the field. J. Chem. Ecol. 6
(1980) 997-1013.
14 Berryman, A.A., Dynamics of bark beetle populations: Towards a general
productivity model. Environ. Entomol. 3 (1974) 579-585.
15 Birgersson, G., and Bergström., Volatiles released from individual spruce bark
beetle entrance holes: quantitative variations during the first week of attack. J.
Chem. Ecol. in press (1989).
16 Birgersson, G., Schlyter, F., Bergström, G., and Löfqvist, J., Individual variation
in aggregation pheromone content of the bark beetle Ips typographus. J.
Chem. Ecol. 14 (1988) 1735-1759.
17 Birgersson, G., Schlyter, F., Löfqvist, J., and Bergström. G., Quantitative
variation of pheromone components in the spruce bark beetle Ips
typographus from different attack phases. J. Chem. Ecol. 99 (1984) 1164-
1193.
18 Birch, M.C., Aggregation in bark beetles. in: W.J. Bell and R.T. Cardé (eds.)
Chemical ecology of insects. (1984) 331-353. Sinauer Associates. Sunderland,
Mass.
19 Birch, M.C., and Wood, D.L., Mutual inhibition of the attractant pheromone
response by two species of Ips (Coleoptera: Scolytidae). J. Chem. Ecol. 1
(1975) 101-113.
20 Birch, M.C., Light, D.M., Wood, D.L., Browne, L.E., Silverstein, R.M., Bergot,
B.J., Ohloff, G., West, J.R., and Young, J.C., Pheromonal attraction and allomonal
interruption of Ips pini in California by the two enantiomers of ipsdienol. J.
Chem. Ecol. 6 (1980) 703-717.
21 Birch, M.C., Svihra, P., Paine, T.D., and Miller, J.C., Influence of chemically
mediated behavior on host tree colonization by four cohabiting species of bark
beetles. J. Chem. Ecol. 6 (1980) 395-414.
22 Borden, J.H., Aggregation pheromones, in: Bark Beetles in North American
Conifers: A System for the Study of Evolutionary Biology, pp. 74-139. Eds J.B.
Mitton and K.B. Sturgeon. Univ. Texas Press, Austin 1982.
23 Borden, J.H., Hunt, D.W.A., Miller, D.R., and Slessor, K.N., Orientation in forest
Coleoptera: An uncertain outcome of responses by individual beetles to variable
stimuli. in, T.L. Payne, M.C. Birch, and C.E.J. Kennedy (eds.). Mechanisms in Insect
Olfaction. Clarendon Press, Oxford. (1986) 97-109.
24 Borden, J.H., Chong, L., McLean, J.A., Slessor, K.N., and Mori, K.,
Gnathotrichus sulcatus: synergistic response to enantiomers of the aggregation
pheromone sulcatol. Science 192 (1976) 894-896.
25 Brand, J.M., and Barras, S.J., The major volatile constituents of a basidiomycete
associated with the southern pine beetle. Lloydia. 40 (1977) 398-400.
26 Brand, J.M., Bracke, J.W., Britton, L.N., Markovetz, A.J., and Barras, J.S., Bark
beetle pheromones: Production of verbenone by a mycangial fungus of
Dendroctonus frontalis. J. Chem. Ecol. 2 (1976) 195-199.
27 Browne, L.E., Wood, D.L., Bedard, W.D., Silverstein, R.M., and West, J.R.,
Quantitative estimates of the Western pine beetle attractive pheromone
components, exo-brevicomin, frontalin, and myrcene in nature. J. Chem.
Ecol. 5 (1979) 397-414.
28 Byers, J.A., Effect of mating on terminating aggregation during host colonization
in the bark beetle, Ips paraconfusus. J. Chem. Ecol. 7 (1981)
1135-1147.
29 Byers, J.A., Pheromone biosynthesis in the bark beetle, Ips paraconfusus,
during feeding or exposure to vapours of host plant precursors. Insect Biochem. 11
(1981) 563-569.
30 Byers, J.A., Male-specific conversion of the host plant compound, myrcene, to
the pheromone, (+)-ipsdienol, in the bark beetle, Dendroctonus brevicomis.
J. Chem. Ecol. 8 (1982) 363-371.
31 Byers, J.A., Influence of sex, maturity and host substances on pheromones in
the guts of the bark beetles, Dendroctonus brevicomis and Ips
paraconfusus. J. Insect Physiol. 29 (1983) 5-13.
32 Byers, J.A., Bark beetle conversion of a plant compound to a sex-specific
inhibitor of pheromone attraction. Science 220 (1983) 624-626.
33 Byers, J.A., Sex-specific responses to aggregation pheromone: Regulation of
colonization density by the bark beetle Ips paraconfusus. J. Chem. Ecol. 9
(1983) 129-142.
34 Byers, J.A., Nearest neighbor analysis and simulation of distribution patterns
indicates an attack spacing mechanism in the bark beetle, Ips typographus
(Coleoptera: Scolytidae). Environ. Entomol. 13 (1984) 1191-1200.
35 Byers, J.A., Interactions of pheromone component odor plumes of western pine
beetle. J. Chem. Ecol. 13 (1987) 2143-2157.
36 Byers, J.A., Upwind flight orientation to pheromone in western pine beetle tested
with rotating windvane traps. J. Chem. Ecol. 14 (1988) 189-198.
37 Byers, J.A., Novel diffusion-dilution method for release of semiochemicals:
Testing pheromone component ratios on western pine beetle. J. Chem. Ecol. 14
(1988) 199-212.
38 Byers, J.A., Behavioral mechanisms involved in reducing competition in bark
beetles. Holarct. Ecol. (1989) in press.
39 Byers, J.A., and Wood, D.L., Interspecific inhibition of the response of the bark
beetles, Dendroctonus brevicomis and Ips paraconfusus. J. Chem.
Ecol. 6 (1980) 149-164.
40 Byers, J.A., and Wood, D.L., Interspecific effects of pheromones on the attraction
of the bark beetles, Dendroctonus brevicomis and Ips paraconfusus
in the laboratory. J. Chem. Ecol. 7 (1981) 9-18.
41 Byers, J.A., and Wood, D.L., Antibiotic-induced inhibition of pheromone synthesis
in a bark beetle. Science 213 (1981) 763-764.
42 Byers, J.A., Anderbrant, O., and Löfqvist, J., Effective attraction radius: A method
for comparing species attractants and determining densities of flying insects. J.
Chem. Ecol. (1988) in press.
43 Byers, J.A., Lanne, B.S., and Löfqvist, J., Host-tree unsuitability recognized by
pine shoot beetles in flight. Experientia (submitted).
44 Byers, J.A., Birgersson, G., Löfqvist, J., and Bergström, G., Synergistic
pheromones and monoterpenes enable aggregation and host recognition by a bark
beetle, Pityogenes chalcographus. Naturwissenschaften 75 (1988) 153-
155.
45 Byers, J.A., Wood, D.L., Craig, J., and Hendry, L.B., Attractive and inhibitory
pheromones produced in the bark beetle, Dendroctonus brevicomis, during
host colonization: Regulation of inter- and intraspecific competition. J. Chem. Ecol.
10 (1984) 861-877.
46 Byers, J.A., Birgersson, G., Appelgren, M., Löfqvist, J., and Bergström, G.,
Isolation of insect pheromone synergists from complex host-plant odors by gas
chromatographic fractionation and subtractive-combination bioassay. J. Chem. Ecol.
(submitted).
47 Byers, J.A., Lanne,2type c:\wp\wpfiles\macro.txt
B.S., Schlyter, F., Löfqvist, J., and Bergström, G., Olfactory recognition of host-tree
susceptibility by pine shoot beetles. Naturwissenschaften 72 (1985) 324-326.
48 Byrne, K.J., Swiger, A.A., Silverstein, R.M., Borden, J.H., and Stokkink, E.,
Sulcatol: population aggregation pheromone in Gnathotrichus sulcatus (Coleoptera:
Scolytidae). J. Insect Physiol. 20 (1974) 1895-1900.
49 Cade, S.C., Hrutfiord, B.F., and Gara, R.I., Identification of a primary attractant
for Gnathotrichus sulcatus isolated from western hemlock logs. J. Econ. Entomol.
3 (1970) 1014-1015.
50 Cardé, R.T., and Baker, T.C. Sexual communication with pheromones, in:
Chemical Ecology of Insects. pp. 355-383. Eds W.J. Bell and R.T. Cardé. Sinauer
Associates, Sunderland, Mass 1984.
51 Choudhury, J.H., and Kennedy, J.S., Light versus pheromone-bearing wind in
the control of flight direction by bark beetles, Scolytus multistriatus. Physiol.
Entomol. 5 (1980) 207-214.
52 Coulson, R.N., Population dynamics of bark beetles. Ann. Rev. Entomol 24
(1979) 417- 447.
53 Dickens, J. C., Behavioural and electrophysiological responses of the bark beetle
Ips typographus to potential pheromone components. Physiol. Entomol. 6
(1981) 251-261.
54 Dickens, J.C., Specificity in perception of pheromones and host odours in
Coleoptera. in: Mechanisms in insect olfaction, (eds.) T.L. Payne, M.C. Birch and
C.E.J. Kennedy. Clarendon Press, Oxford. (1986) 253-261.
55 Dickens, J.C., Payne, T.L., Ryker, L.C., and Rudinsky, J.A., Multiple acceptors
for pheromonal enantiomers on single olfactory cells in the Douglas-fir beetle,
Dendroctonus pseudotsugae Hopk. (Coleoptera: Scolytidae) J. Chem. Ecol. 11
(1985) 1359-1370.
56 Doskotch, R.W., Chatterji, S.K., and Peacock, J.W., Elm bark derived feeding
stimulants for the smaller European elm bark beetle. Science 167 (1970)
380-382.
57 Elkinton, J.S., and Wood, D.L., Feeding and boring behavior of the bark beetle
Ips paraconfusus (Coleoptera: Scolytidae) on the bark of a host and non-host
tree species. Can. Entomol. 112 (1980) 797-809.
58 Elkinton, J.S., Wood, D.L., and Browne, L.E., Feeding and boring behavior of the
bark beetle, Ips paraconfusus, in extracts of ponderosa pine phloem. J.
Chem. Ecol. 7 (1981) 209-220.
59 Francke, W., and Vité, J.P., Oxygenated terpenes in pheromone systems of bark
beetles. Z. Angew. Entomol. 96 (1983) 146-156.
60 Francke, W., Sauerwein, P., Vité, J.P., and Klimetzek, D., The pheromone
bouquet of Ips amitinus. Naturwissenschaften 67 (1980) 147-148.
61 Francke, W., Heemann, V., Gerken, B., Renwick, J.A.A., and Vité, J.P.,
2-ethyl-1-6-dioxaspiro[4.4]nonane, principal aggregation pheromone of
Pityogenes chalcographus (L.). Naturwissenschaften 64 (1977)
590-591.
62 Furniss, M.M., Baker, B.H., and Hostetler, B.B., Aggregation of spruce beetles
(Coleoptera) to seudenol and repression of attraction by methylcyclohexenone in
Alaska. Can. Entomol. 108 (1976) 1297-1302.
63 Gilbert, B.L., Baker, J.E., and Norris, D.M., Juglone (5-hydroxy-1,
4-napthoquinone) from Carya ovata, a deterrent to feeding by Scolytus
multistriatus. J. Insect Physiol. 13 (1967) 1453-1459.
64 Goeden, R.D., and Norris, D.M., Attraction of Scolytus quadrispinosus
(Coleoptera: Scolytidae) to Carya spp. for oviposition. Ann. Entomol. Soc. Am. 57
(1964) 141-146.
65 Graham, K., Release by flight exercise of a chemotropic response from
photopositive domination in a scolytid beetle. Nature 184 (1959) 283-284.
66 Gregoire, J.C, Broekman, J.C., and Tondeur, A., Chemical communication
between the larvae of Dendroctonus micans Kug. (Coleoptera, Scolytidae).
Med. Chem. Agis. Comp. Insectes 7 (1982) 253-257.
67 Hedden, R.L., and Gara, R.I., Spatial attack pattern of a western Washington
Douglas-fir beetle population. For. Sci. 22 (1976) 100-102.
68 Hodges, J.D., Elam, W.W., Watson, W.R., and Nebeker, T.E., Oleoresin
characteristics and susceptibility of four southern pines to southern pine beetle
(Coleoptera: Scolytidae) attacks. Can. Entomol. 111 (1979) 889-896.
69 Hughes, P.R., Myrcene: a precursor of pheromones in Ips beetles. J.
Insect Physiol. 20 (1974) 1271-1275.
70 Hunt, D.W.A., Borden, J.H., Pierce, H.D., Jr., Slessor, K.N., King, G.G.S., and
Csyzewska, E.K., Sex-specific production of ipsdienol and myrcenol by
Dendroctonus ponderosae (Coleoptera: Scolytidae) exposed to myrcene
vapors. J. Chem. Ecol. 12 (1986) 1579-1586.
71 Hynum, B.G., and Berryman, A.A., Dendroctonus ponderosae
(Coleoptera: Scolytidae): pre-aggregation landing and gallery initiation on lodgepole
pine. Can. Entomol. 112 (1980) 185-191.
72 Kennedy, J.S., Some current issues in orientation to odour sources, in:
Mechanisms in Insect Olfaction. pp. 11-25. Eds T.L Payne, M.C. Birch and C.E.J.
Kennedy. Clarendon Press, Oxford 1986.
73 Kinzer, G.W., Fentiman, A.F., Jr., Page, T.F., Foltz, R.L., Vité, J.P., and Pitman,
G.B., Bark beetle attractants: identification, synthesis and field bioassay of a new
compound isolated from Dendroctonus. Nature 211 (1969) 475-476.
74 Kirkendall, L.R., The evolution of mating systems in bark and ambrosia beetles
(Coleoptera: Scolytidae and Platypodidae). Zool. J. Linn. Soc. 77 (1983) 293-
352.
75 Klimetzek, D., and Francke, W., Relationship between the enantiomeric
composition of o-pinene in host trees and the production of verbenols in Ips
species. Experientia 36 (1980) 1343-1345.
76 Klimetzek, D., Köhler, J., Vité, J.P., and Kohnle, U., Dosage response to ethanol
mediates host selection by 'secondary' bark beetles. Naturwissenschaften 73 (1986)
270-272.
77 Krebs, C.J., Ecology. (1972) 3-4, Harper and Row, New York. 694 p.
78 Lanier, G. N., Integration of visual stimuli, host odorants, and pheromones by
bark beetles and weevils in locating and colonizing host trees, in: Herbivorous
Insects: Host-Seeking Behavior and Mechanisms. pp. 161-171. Ed S. Ahmad.
Academic Press, New York 1983.
79 Lanier, G.N., Classon, A., Stewart, T., Piston, J.J., and Silverstein, R.M., Ips pini:
the basis for interpopulational differences in pheromone biology. J. Chem. Ecol. 6
(1980) 677-687.
80 Lanne, B.S., Schlyter, F., Byers, J.A., Löfqvist, J., Leufvén, A., Bergström, G. ,
Van Der Pers, J.N.C., Unelius, R., Baeckström, P., and Norin, T., Differences in
attraction to semiochemicals present in sympatric pine shoot beetles, Tomicus
minor and T. piniperda. J. Chem. Ecol. 13 (1987) 1045-1067.
81 Leufvén, A., and Birgersson, G., Quantitative variation of different monoterpenes
around galleries of Ips typographus (Coleoptera: Scolytidae) attacking
Norway spruce. Can. J. Bot. 65 (1987) 1038-1044.
82 Leufvén, A., Bergström, G., and Falsen, E., Interconversion of verbenols and
verbenone by identified yeasts isolated from the spruce bark beetle Ips
typographus. J. Chem. Ecol. 10 (1984) 1349-1361.
83 Libbey, L.M., Morgan, M.E., Putnam, T.B., and Rudinsky, J.A., Isomer of
antiaggregative pheromone identified from male Douglas-fir beetle:
3-methylcyclohexen-1-one. J. Insect Physiol. 22 (1976) 871-873.
84 Light, D.M., and Birch, M.C., Inhibition of the attractive pheromone response in
Ips paraconfusus by (R)-(-)-ipsdienol. Naturwissenschaften 66 (1979)
159.
85 Light, D.M., and Birch, M.C., Bark beetle enantiomeric chemoreception: Greater
sensitivity to allomone than pheromone. Naturwissenschaften 69 (1982)
243-245.
86 Lorio, P.L. Jr., and Hodges, J.D., Oleoresin exudation pressure and relative
water content of inner bark as indicators of moisture stress in loblolly pines. For.
Sci. 14 (1968) 392-398.
87 MacConnell, J.G., Borden, J.H., Silverstein, R.M., and Stokkink, E., Isolation and
tentative identification of lineatin, a pheromone from the frass of Trypodendron
lineatum (Coleoptera: Scolytidae). J. Chem. Ecol. 3 (1977) 549-561.
88 Mayyasi, A.M., Coulson, R.N., Foltz, J.L., Hain, F.P., and Martin, W.C.,
Functional description of within-tree larval and progeny adult populations of
Dendroctonus frontalis (Coleoptera: Scolytidae). Can. Entomol. 108 (1976)
363-372.
89 Miller, J.M., and Keen, F.P., Biology and control of the western pine beetle. U.S.
Dept. Agric. Misc. Publ. No. 800.
90 Moeck, H.A., Ethanol as the primary attractant for the ambrosia beetle
Trypodendron lineatum (Coleoptera: Scolytidae). Can. Entomol. 102 (1970)
985-995.
91 Moeck, H.A., Field test for the primary attraction of the spruce beetle. Environ.
Can. For. Serv. Bi-mon. Res. Notes 34 (1978) 8.
92 Moeck, H.A., Wood, D.L., and Lindahl, K.Q.Jr., Host selection behavior of bark
beetles (Coleoptera: Scolytidae) attacking Pinus ponderosa, with special
emphasis on the western pine beetle, Dendroctonus brevicomis. J. Chem.
Ecol. 7 (1981) 49-83.
93 Murlis, J., The structure of odour plumes, in: Mechanisms in Insect Olfaction. pp.
27- 38. Eds T.L. Payne, M.C. Birch and C.E.J. Kennedy. Clarendon Press, Oxford
1986.
94 Mustaparta, H., Angst, M.E., and Lanier, G.N., Receptor discrimination of
enantiomers of the aggregation pheromone ipsdienol, in two species of Ips.
J. Chem. Ecol. 6 (1980) 689-701.
95 Mustaparta, H., Tommerĺs, B.Ä., Baeckström, P., Bakke, J.M., and Ohloff, G.,
Ipsdienol-specific receptor cells in bark beetles: Structure-activity relationships of
deuterium-labelled ipsdienol. J. Comp. Physiol. 154 (1984) 591-595.
96 Nilssen, A.C., Spatial attack pattern of the bark beetle Tomicus piniperda
L. (Col., Scolytidae). Norw. J. Entomol. 25 (1978) 171-175.
97 Norris, D.M., Role of repellents and deterrents in feeding of Scolytus
multistriatus. in: Host Plant Resistance to Pests, pp. 215-230. Ed P.A. Hedin.
Am. Chem. Symp. Ser. No. 63, 1977.
98 Paine, T.D., Birch, M.C., and Svihra, P., Niche breadth and resource partitioning
by 4 sympatric species of bark beetles. Oecologia 48 (1981) 1-6.
99 Payne, T.L., Pheromone and host odor perception in bark beetles, in:
Neurotoxicology of Insecticides and Pheromones, pp. 27-57. Ed T. Narahashi.
Plenum Pub. Co., New York 1979.
100 Payne, T.L., and Dickens, J.C. Adaptation to determine receptor system
specificity in insect olfactory communication. J. Insect Physiol. 22 (1976)
1569-1572.
101 Payne, T.L., Klimetzek, D., Kohnle, U., and Mori, K., Electro-physiological and
field responses of Trypodendron-spp to enantiomers of lineatin. Z. Angew. Entomol.
95 (1983) 272-276.
102 Payne, T.L., Richerson, J.V., Dickens, J.C., West, J.R., Mori, K., Berisford, C.
W., Hedden, R.L., Vité, J.P., and Blum, M.S., Southern pine beetle: Olfactory
receptor and behavior discrimination of enantiomers of the attractant pheromone
frontalin. J. Chem. Ecol. 8 (1982) 873-881.
103 Peacock. J.W., Lincoln, C.L., Simeone, J.B., and Silverstein, R.M., Attraction
of Scolytus multistriatus (Coleoptera: Scolytidae) to a virgin-female-produced
pheromone in the field. Ann. Entomol. Soc. Am. 64 (1971) 1143-1149.
104 Pearce, G.T., Gore, W.E., Silverstein, R.M., Peacock, J.W., Cuthbert, R.A.,
Lanier, G.N., and Simeone, J.B., Chemical attractants for the smaller European elm
bark beetle, Scolytus multistriatus (Coleoptera: Scolytidae). J. Chem. Ecol.
1 (1975)
115-124.
105 Peschke, K., Cuticular hydrocarbons regulate mate recognition, male
aggression, and female choice of the rove beetle, Aleochara curtula. J.
Chem. Ecol. 13 (1987) 1993-2008.
106 Pitman, G.B., and Vité, J.P., Biosynthesis of methylcyclohexenone by male
Douglas-fir beetle. Environ. Entomol. 3 (1974) 886-887.
107 Pitman, G.B., Vité, J.P., Kinzer, G.W., and Fentiman, A.F., Jr., Bark beetle
attractants: trans-verbenol isolated from Dendroctonus. Nature 218 (1968)
168-169.
108 Raffa, K.F., and Berryman, A.A.,. Physiological aspects of lodgepole pine
wound responses to a fungal symbiont of the mountain pine beetle,
Dendroctonus ponderosae (Coleoptera: Scolytidae). Can. Entomol. 115
(1983) 723-734.
109 Renwick, J.A.A., and Vité, J.P., Systems of chemical communication in
Dendroctonus. Contrib. Boyce Thompson Inst. 24 (1970) 283-292.
110 Renwick, J.A.A., Hughes, P.R., and Krull, I.S., Selective production of cis- and
trans-verbenol from (-)- and (+)-a-pinene by a bark beetle. Science 191 (1976)
199-201.
111 Renwick, J.A.A., and Dickens, J.C., Control of pheromone production in the
bark beetle, Ips cembrae. Physiol. Entomol. 4 (1979) 377-381.
112 Rudinsky, J.A., Multiple functions of the Douglas-fir beetle pheromone,
3-methyl-2-cyclohexen-1-one. Environ. Entomol. 2 (1973) 579-585.
113 Rudinsky, J.A., and Michael, R.R., Sound production in Scolytidae: Stridulation
by female Dendroctonus beetles. J. Insect Physiol. 19 (1973) 689-705.
114 Rudinsky, J.A., and Ryker, L.C., Sound production in Scolytidae: rivalry and
premating stridulation of male Douglas-fir beetle. J. Insect Physiol. 22 (1976) 997-
1003.
115 Rudinsky, J.A., Morgan, M.E., Libbey, L.M., and Michael, R.R., Sound
production in Scolytidae: 3-methyl-2-cyclohexen-1-one released by the female
Douglas-fir beetle in response to male sonic signal. Environ. Entomol. 2 (1973)
505-509.
116 Rudinsky, J.A., Morgan, M.E., Libbey, L.M., and Putnam, T.B., Additional
components of the Douglas-fir beetle (Col., Scolytidae) aggregative pheromone and
their possible utility in pest control. Z. Angew. Entomol. 76 (1974) 65-77.
117 Ryker, L.C., Acoustic and chemical signals in the life cycle of a beetle. Sci. Am.
250 (1984) 112-115,118-123,154
118 Safranyik, L., and Vithayasai, C., Some characteristics of the spatial
arrangement of attacks by the mountain pine beetle, Dendroctonus ponderosae
(Coleoptera: Scolytidae), on lodgepole pine. Can. Entomol. 103 (1971)
1607-1625.
119 Schlyter, F., Birgersson, G., and Leufvén, A., Inhibition of attraction to
aggregation pheromone by verbenone, ipsenol and ipsdienol: Density regulation
mechanisms in the bark beetle Ips typographus. J. Chem. Ecol. in press
(1989).
120 Schlyter, F., Byers, J.A., and Löfqvist, J.A., Attraction to pheromone sources of
different quantity, quality and spacing: Density-regulation mechanisms in the bark
beetle Ips typographus. J. Chem. Ecol. 13 (1987) 1503-1523.
121 Schlyter, F., Löfqvist, J., and Byers, J.A., Behavioural sequence in the attraction
of the bark beetle Ips typographus to pheromone sources. Physiol. Entomol.
12 (1987) 185-196.
122 Schlyter, F., Birgersson, G., Byers, J.A., Löfqvist, J., and Bergström, G.,
Response of spruce bark beetle, Ips typographus, to aggregation pheromone
candidates. J. Chem. Ecol. 13 (1987) 701-716.
123 Schurig, V., and Weber, R., Use of glass and fused-silica open tubular columns
for the separation of structural, configurational and optical isomers by selective
complexation gas chromatography. J. Chromatog. 289 (1984) 321-332.
124 Shepherd, R.F., Distribution of attacks by Dendroctonus ponderosae Hopk. on
Pinus contorta Dougl. var. latifolia Engelm. Can. Entomol. 97 (1965) 207-215.
125 Shrimpton, D.M., Resistance of lodgepole pine to mountain pine beetle
infestation (1978) 64-76. in, Theory and practice of mountain pine beetle
management in lodgepole pine forests. Forest, Wildlife and Range Exp. Sta. Univ.
Idaho,Moscow, Idaho 224 pp. 126 Silverstein, R.M., Rodin, J.O., and Wood, D.L.,
Sex attractants in frass produced by male Ips confusus in ponderosa pine.
Science 154 (1966) 509-510.
127 Silverstein, R.M., Rodin, J.O., and Wood, D.L., Methodology for isolation and
identification of insect pheromones with reference to studies on California
five-spined Ips. J. Econ. Entomol. 60 (1967) 944-949.
128 Silverstein, R.M., Rodin, J.O., Wood, D.L., and Browne, L.E., Identification of
two new terpene alcohols from frass produced by Ips confusus in ponderosa
pine. Tetrahedron 22 (1966) 1929-1936.
129 Silverstein, R.M., Brownlee, R.G., Bellas, T.E., Wood, D.L., and Browne, L.E.,
Brevicomin: Principal sex attractant in the frass of the female western pine beetle.
Science 159 (1968) 889-891.
130 Slessor, K.N., King, G.G.S., Miller, D.R., Winston, M.L., and Cutforth, T.L.,
Determination of chirality of alcohol or latent alcohol semiochemicals in individual
insects. J. Chem. Ecol.
11 (1985) 1659-1667.
131 Smith, R.H., The fumigant toxicity of three pine resins to Dendroctonus
brevicomis and D. jeffrei. J. Econ. Entomol. 54 (1961) 365-369.
132 Smith, R.H.,. Variation in the monoterpenes of Pinus ponderosa Laws.
Science 143 (1964) 1337-1338.
133 Smith. R.H., Local and regional variation in the monoterpenes of ponderosa
pine xylem resin. USDA For. Ser. Res. Pap. PSW-56 (1969) 1-10.
134 Stephen, F.M., and Dahlsten, D.L., The arrival sequence of the arthropod
complex following attack by Dendroctonus brevicomis (Coleoptera:
Scolytidae) in ponderosa pine. Can. Entomol. 108 (1976) 283-304.
135 Sturgeon, K.B., Monoterpene variation in ponderosa pine xylem resin related
to western pine beetle predation. Evolution 33 (1979) 803-814.
136 Svihra, P., Paine, T.D., and Birch, M.C., Interspecific olfactory communications
in southern pine beetles. Naturwissenschaften 67 (1980) 518.
137 Tommerĺs, B.Ä., Mustaparta, H., and Gregoire, J.C., Receptor cells in Ips
typographus and Dendroctonus micans specific to pheromones of the reciprocal
genus. J. Chem. Ecol. 10 (1984) 759-769.
138 Vanderwel, D., and Oehlschlager, A.C., Biosynthesis of pheromones and
endocrine regulation of pheromone production in Coleoptera, in: Pheromone
Biochemistry. pp. 175-215. Eds G.D. Prestwich and G.J. Blomquist. Academic
Press, New York 1987.
139 Vité, J.P., and Gara, R.I., Volatile attractants from ponderosa pine attacked by
bark beetles (Coleoptera: Scolytidae). Contrib. Boyce Thompson Inst. 21 (1962)
251-273.
140 Vité, J.P., and Williamson, D.L., Thanasimus dubius: prey perception. J. Insect
Physiol. 16 (1970) 233-239.
141 Vité, J.P., and Wood, D.L., A study on the applicability of the measurement of
oleoresin exudation pressure in determining susceptibility of second growth
ponderosa pine to bark beetle infestation. Contrib. Boyce Thompson Inst. 21 (1961)
67-78.
142 Vité, J.P., Bakke, A., and Renwick, J.A.A., Pheromones in Ips (Coleoptera:
Scolytidae): Occurrence and production. Can. Entomol. 104 (1972) 1967-1975.
143 Vité, J.P., Hedden, R., and Mori, K., Ips grandicollis: field response to the
optically pure pheromone. Naturwissenschaften 63 (1976) 43-44.
144 Vité, J.P., Ohloff, C., and Billings, R.F., Pheromone chirality and integrity of
aggregation response in southern species of the bark beetle, Ips sp. Nature 272
(1978) 817-818.
145 Vité, J.P., Pitman, G.B., Fentiman, A.F.Jr., and Kinzer, G.W.,
3-Methyl-2-cyclohexen-1-ol isolated from Dendroctonus. Naturwissenschaften 59
(1972) 469.
146 Wood, D.L., Selection and colonization of ponderosa pine by bark beetles, in:
Insect Plant Relationships. pp. 101-117. Ed H.F. van Emden. Blackwell Sci. Pub.,
London 1972.
147 Wood, D.L., The role of pheromones, kairomones, and allomones in the host
selection and colonization behavior of bark beetles. Ann. Rev. Entomol. 27 (1982)
411-446.
148 Wood, D.L., Akers, R.P., Owen, D.R., and Parmeter, J.R., Jr., The behaviour
of bark beetles colonizing ponderosa pine, in: Insects and the Plant Surface. pp. 91-
104. Eds Juniper and Southwood. Arnold Publ., London 1986.
149 Wood, D.L., Browne, L.E., Silverstein, R.M., and Rodin, J.O., Sex pheromones
of bark beetles - I. Mass production, bioassay, source, and isolation of the sex
pheromone of Ips confusus (LeC.). J. Insect Physiol. 12 (1966)
523-536.
150 Wood, D.L., Stark, R.W., Silverstein, R.M., and Rodin, J.O., Unique synergistic
effects produced by the principal sex attractant compounds of Ips confusus
(LeConte)(Coleoptera: Scolytidae). Nature
215 (1967) 206.
151 Wood, D.L., Browne, L.E., Ewing, B., Lindahl, K., Bedard, W.D., Tilden,P.E.,
Mori, K., Pitman, G.B., and Hughes, P.R., Western pine beetle: specificity among
enantiomers of male and female components of an attractant pheromone. Science
192 (1976) 896-898.
152 Wood, S.L., The bark and ambrosia beetles of North and Central America
(Coleoptera: Scolytidae), a taxonomic monograph. Great basin naturalist memoirs,
Brigham Young Univ., Provo, Utah. (1982) 1359 p.






