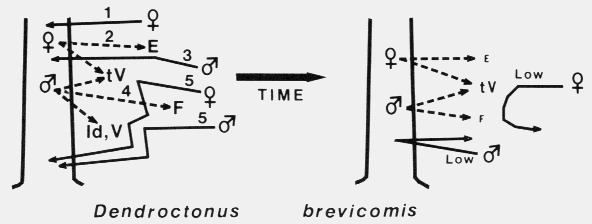
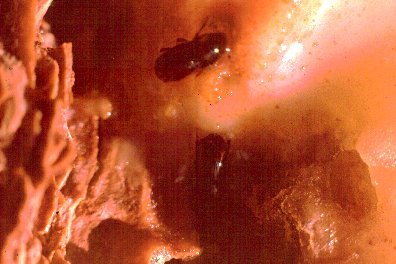
 A female western pine beetle, Dendroctonus brevicomis, in upper center is about to reenter the resin-filled
hole leading to the phloem. A male is at center bottom and is having trouble climbing back up. He helps the
female with removing frass and resin. One of the most well-adapted bark beetles to host resin.
A female western pine beetle, Dendroctonus brevicomis, in upper center is about to reenter the resin-filled
hole leading to the phloem. A male is at center bottom and is having trouble climbing back up. He helps the
female with removing frass and resin. One of the most well-adapted bark beetles to host resin.
 Here I am climbing the actual ponderosa pine used in the study here.
Here I am climbing the actual ponderosa pine used in the study here.
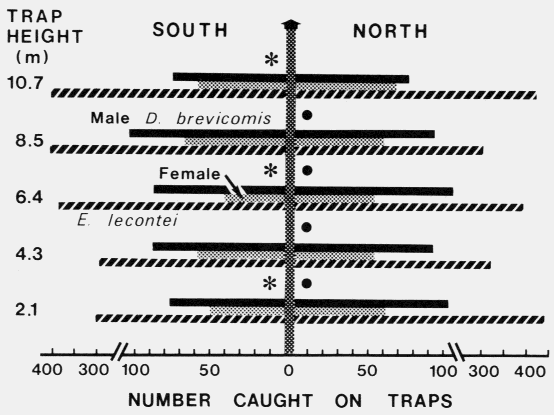
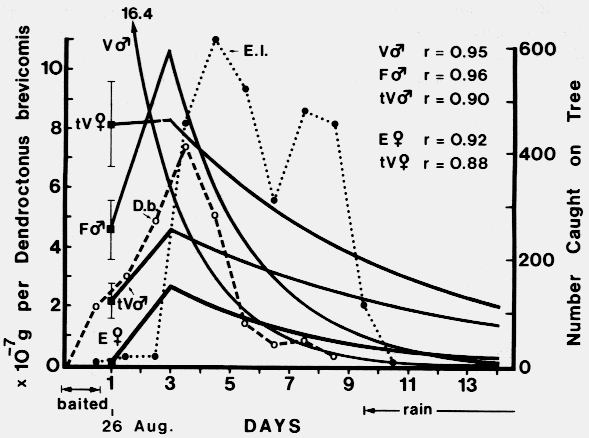
| TABLE 1. Relationships between aggregation of Dendroctonus brevicomis and Enoclerus lecontei on ponderosa pine and male stridulation, egg maturation, and gallery length of D. brevicomis, and host resin exudation (August - September 1978). | ||||||
| Catch on traps) | ||||||
|---|---|---|---|---|---|---|
| Days after initial attack | Trap catch male:female | Male stridulation | Gravid females | Range of gallery lengths (cm) | E. lecontei | Resin exudation |
| Aug. 24 | 0 | 0 | 0 | 0 | 0 | 0 |
| Aug. 25a | 57:34 | ++b | 0 | 0 | 5 | 0 |
| Day 1 | 97:53 | +++ | 0 | *d | 15 | 0 |
| Day 2 | 151:120 | +++ | 0 | * | 15 | +e |
| Day 3 | 255:153 | +++ | +c | * | 455 | +++ |
| Day 4 | 172:111 | ++ | +++ | 0.6-2.5 | 614 | +++ |
| Day 5 | 54:27 | ++ | +++ | 1.2-3.8 | 519 | + |
| Day 6 | 25:15 | + | +++ | 2.0-5.1 | 302 | 0 |
| Day 7 | 29:18 | + | +++ | 2.5-6.2 | 483 | 0 |
| Day 11 | rain | 0 | +++ | < 9.0 | rain | 0 |