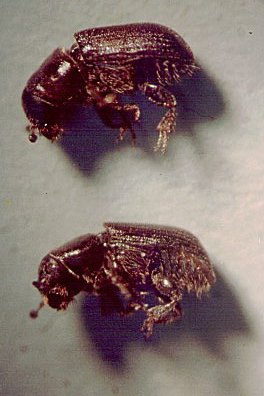
 Sticky-traps for catching Dendroctonus brevicomis, the western pine beetle, in ponderosa pine forest habitat
near Oakhurst (and Yosemite), California.
Sticky-traps for catching Dendroctonus brevicomis, the western pine beetle, in ponderosa pine forest habitat
near Oakhurst (and Yosemite), California.
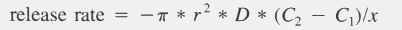
 ( 1 )
( 1 )
| mls = fws * ( gsem / fwsem - fsem * gsem / fwsem ) / fsem / gs |
|---|
| Table 1. Catches of male and female Dendroctonus brevicomis (and sex ratios) and Temnochila chlorodia on sticky traps releasing various ratios of ethanol (A), frontalin (F), exo-brevicomin (E), and myrcene (M). | |||||
| D. brevicomis | T. chlorodia | ||||
|---|---|---|---|---|---|
| Component release ratesa | Male | Female | M/F sex ratio (95% BCL)b | Male | Female | Ac | 0 | 0 | 0 | 0 | F + M + Ac | 6 | 10 | 0.60 (0.23 - 1.59) | 0 | 0 | E + M + Ac | 8 | 3 | 2.67 (0.77 - 9.26) | 4 | 2 | 0.01 F (in Ad) + E + M | 53**e | 57* | 0.93 (0.64 - 1.35) | 5 | 4 | 0.01 E (in Ac) + F + M | 60** | 68** | 0.88 (0.62 - 1.25) | 0 | 0 | 0.1 F (in Ad) + E + M | 98 | 107** | 0.92 (0.70 - 1.20) | 4 | 7 | 0.1 E (in Ac) + F + M | 124** | 158** | 0.78 (0.62 - 0.99) | 2 | 1 | E + F + M + Ac | 155 | 170 | 0.91 (0.73 - 1.13) | 5 | 4 |
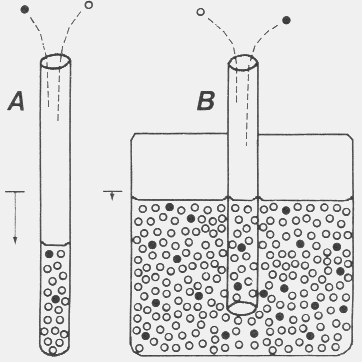
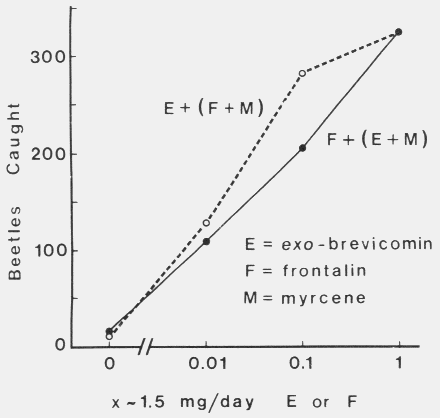
|
JOHN A. BYERS Department of Animal Ecology, University of Lund, S-223 62 Lund, Sweden Department of Entomological Sciences, University of California Berkeley, California 94720 Present address: 
|
|---|